“If we look at major trends, people are living longer, on average, so there’s a rise in chronic diseases associated with aging. Meanwhile, healthcare workers are in short supply, and physicians and nurses do not have enough time for patients. Yet everyone has powerful computers in their pockets that give them access to technology, education, and information. Put all that together, and it’s the perfect moment for digital solutions to come to the market, change behavior, and enhance health outcomes at scale”.
–Bozidar Jovicevic, Global head of Digital Therapeutics, Sanofi

The healthcare industry is facing a crisis in treating chronic illness. Coronavirus has shed light on the urgency of traditional healthcare partnering with digital health solutions.
Introduction to digital therapeutics
The Digital Therapeutic Alliance defines digital therapeutics (DTx) as evidence-based therapeutic interventions delivered to patients that are driven by high quality software programs to prevent, manage, or treat a medical disorder or disease.
They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes.
The Digital Therapeutics Alliance (DTA) headquartered in the US was founded in 2017. It is a non-profit trade association of industry leaders and stakeholders engaged in the evidence driven advancement of DTx.
Sanofi has a digital therapeutics division that covers:
– Prescription Digital Therapeutics
– Patient monitoring and engagement
– Disease and medication management
– Telemedicine
The global DTx market has been growing significantly and at present is valued at USD 2.1 billion and is projected to reach USD 6.9 billion by 2025. Digital solutions are also an opportunity for growth which is reflected in the total investment in digital therapeutics, which to date has topped $600 million.
DTx can solve many problems that plague patients trying to access healthcare
Healthcare organizations must investigate convenient care options and other patient services to drive more patient access to healthcare. When a patient cannot access her clinician, it is impossible to receive medical care, build relationships with her providers, and achieve overall patient wellness. Between appointment availability issues and troubles getting a ride to the clinician office, patient care access has many associated challenges like:

LIMITED APPOINTMENT AVAILABILITY, OFFICE HOURS
Many healthcare organizations offer a typical set of office hours for patient visits. But for the working adult or parent, a clinic that is open between 8 a.m. and 6 p.m. is not always useful. Patients need convenient office hours that allow them to visit the doctor outside of their work or school schedules.
GEOGRAPHIC, CLINICIAN SHORTAGE ISSUES
Patients living in rural areas are disproportionately more likely to struggle to access their clinician than a patient living in an urban or suburban area. The patient-to-primary care physician ratio in rural areas is 39.8 physicians per 100,000 people, compared to 53.3 physicians per 100,000 in urban areas, according to statistics from the National Rural Health Association.
TRANSPORTATION BARRIERS
Patients who are physically unable to drive, who face financial barriers, or who otherwise cannot obtain transportation to the clinician office often go without care.
LIMITED EDUCATION ABOUT CARE SITES
Oftentimes, patient care access issues are not about getting a foot in the door. Instead, it’s about getting a foot in the right door. For example, only 46 percent of respondents correctly selected urgent care as the appropriate choice for a scenario in which a child is presenting with 104-degree fever, shivering, and coughing.
Digital therapeutics and the patient health care journey
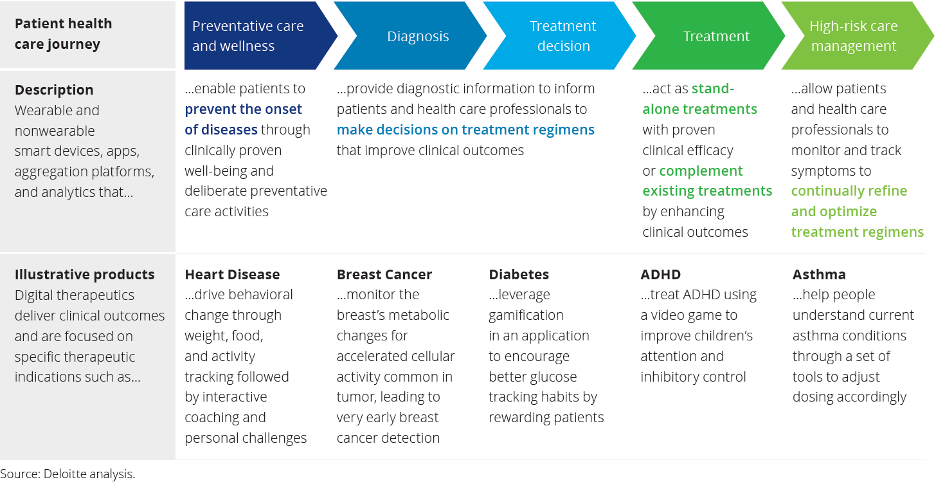
Core principles all digital therapeutics must adhere to:
- Prevent, manage, or treat a medical disorder or disease
- Produce a medical intervention that is driven by software, and delivered via software or complementary hardware, medical device, service, or medication
- Incorporate design, manufacture, and quality best practices
- Incorporate patient privacy and security protections
- Be reviewed and cleared or approved by regulatory bodies as required to support product claims of risk, efficacy, and intended use
- Make claims appropriate to clinical validation and regulatory status
- Collect, analyze, and apply real world evidence and product
- Performance data
Benefits of Digital Therapeutics
1. For patients and caregivers:
- Deliver reliable, evidenced-based interventions with a high control of quality
- Increase access to therapies that are clinically demonstrated as safe and effective
2. For healthcare providers and health systems:
- Increase access to novel treatment options for patients with unmet medical needs
- Integrate into healthcare delivery systems in accordance with industry best practices and guidelines.
Industry-adopted best practices for digital therapeutics include:
- Product Design, Development, Manufacture
- Clinical Validation
- Product Security and Maintenance
- Regulatory Oversight
Digital Therapeutics Ecosystem
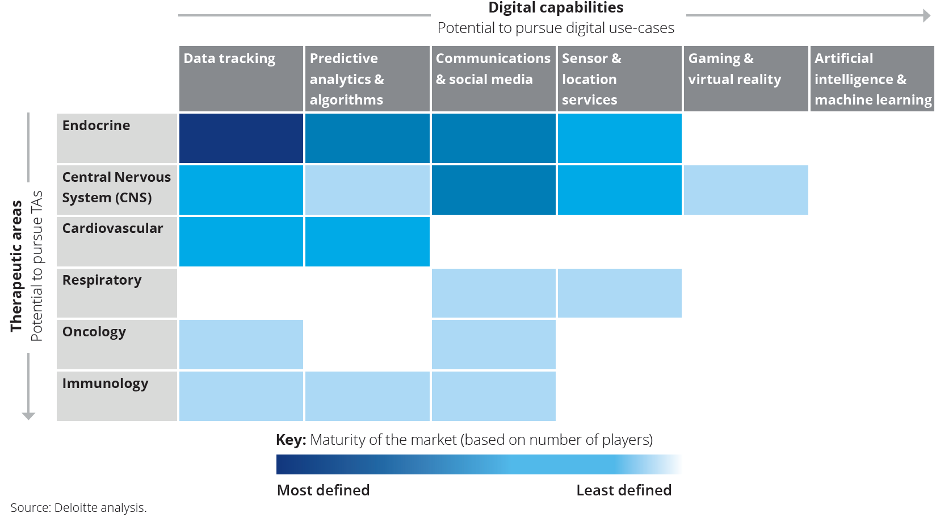
The Digital Therapeutics Alliance adopted a four-category classification of DTx. Based on its target product profile, each DTx treatment belongs to one of the following categories:
1. Address a medical condition.
2. Manage or prevent a medical disorder or disease.
3. Optimize medication.
4. Treat a medical disease or disorder.
Regulatory Process or Guidelines for Digital Therapeutics
The world’s first prescription DTx was developed and approved in the US. DTx is regulated by the Center for Devices and Radiological Health (CDRH) at the FDA.
The FDA considers DTx submission as mobile medical applications (MMA). MMAs that do not fall under this enforcement discretion policy are classified and regulated as Class I, Class II, or Class III devices, depending on an analysis of safety/risk, intended use, and indications for use.
For Class II and I devices categorized as “special” and “general” controls respectively, a 510(k) premarket submission is required.
Class III is designated for devices possessing the most significant risk of illness or injury, and these devices are subject to the premarket approval (PMA) review process.
A 510(k) compares the device to one or more similar devices that are already legally marketed and claims the new device to be substantially equivalent. If no such marketed devices exist, a de novo submission for a Class I or II device must be made.
For example, Pear Therapeutics’ reSET® for the treatment of substance use disorder was approved through a de novo submission for a Class II medical device. Also, their reSET-OTM went through the expedited access pathway and was reviewed under a 510(k), claiming substantial equivalence to the already approved reSET®.
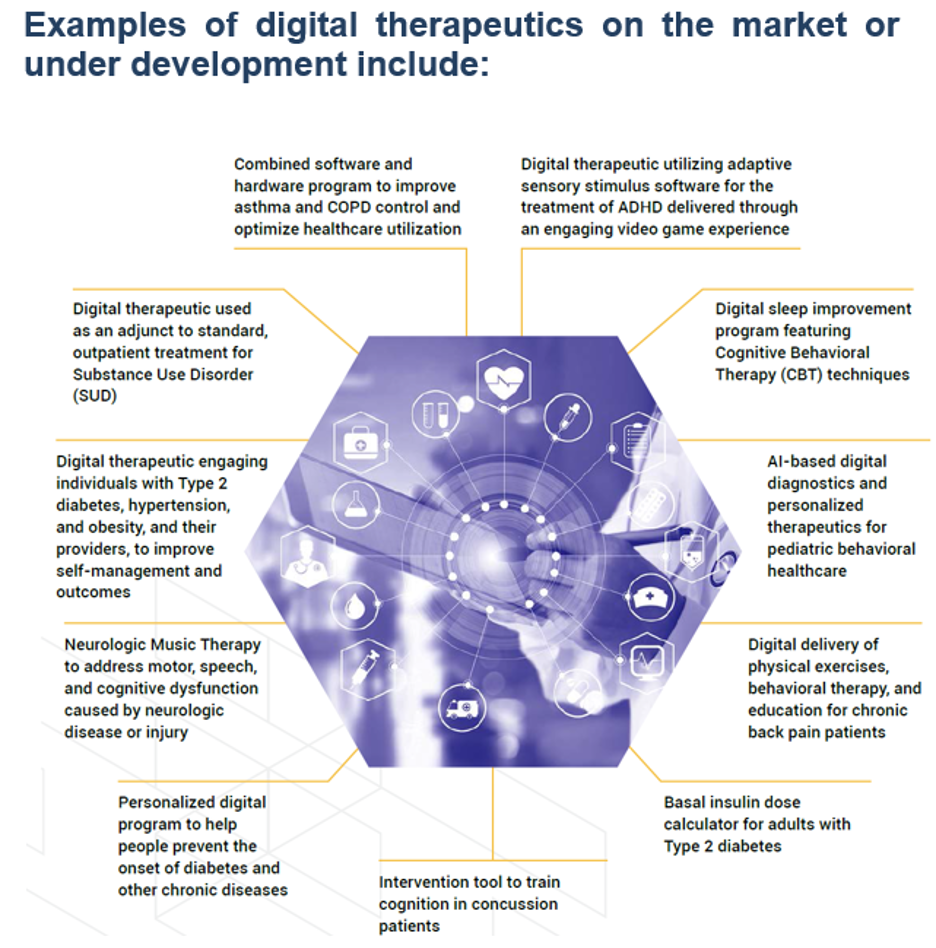
Recent FDA Marketed Approval in Digital Therapeutics
1. BiovitalsHF
Prescription software to enable early detection of heart failure exacerbation and augment guideline-directed use of heart failure medications.
FDA 510(k) clearance for Biovitals Analytics Engine and market Authorization in the U.S. for BiovitalsHF VI.
2. Rhythm Analytics
Cloud based software for automated detection of over 15 types of cardiac arrhythmias.
FDA 510(k) clearance for Rhythm Analytics – A Software as a Service (SaaS) offering with scalable APIs to enable easy integration.
A significant number of therapies in development for CNS indications due to the ability to impact neuroplasticity and behavior-mediated indications:

Key Players for delivering Digital Therapeutics – A Closer Look at Three Digital Therapy Companies
1. Pear Therapuetics
Discovers, develops, and delivers clinically-validated software-based therapeutics to provide better outcomes for patients, smarter engagement and tracking tools for clinicians, and cost-effective solutions for payers.
Two Marketed Products:
reSET is an FDA-cleared 12-week interval prescription therapeutic for Substance Use Disorder intended to provide cognitive behavioral therapy, as an adjunct to standard, outpatient treatment.
reSET-O is an 84-day Prescription Digital Therapeutic (PDT) for Opioid Use Disorder intended to increase retention of patients in outpatient treatment by providing cognitive behavioral therapy, as an adjunct to outpatient treatment that includes transmucosal buprenorphine and contingency management.
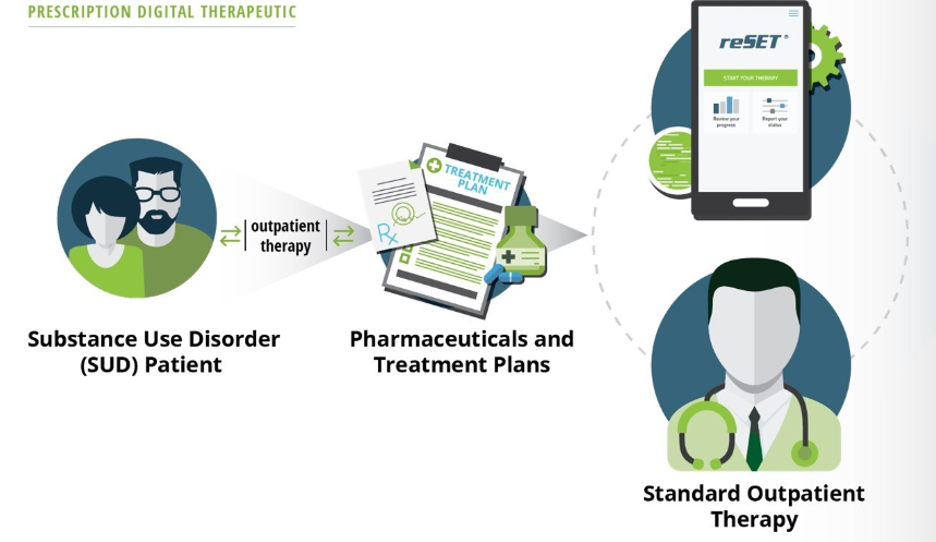
An associated dashboard for clinicians and other health care providers can be used as part of treatment for both products. The dashboard displays information about patients’ use of the product, including lessons completed, patient-reported substance use, patient-reported cravings and triggers, patient-reported medication use, compliance rewards, and in-clinic data inputs such as urine drug screen results.
Over 10 products in development across therapeutic areas, including insomnia, depression, schizophrenia, IBS, pain, PTSD, migraine, bipolar disorder, multiple sclerosis, epilepsy and various oncology indication
2. Propeller Health
Digital therapeutics company providing connected health solutions for people with COPD and asthma.
Uses sensors with inhalers to track medication usage and provide personal feedback and insights that enables integration of care through the progression of the disease.

Platform is used by patients, providers and healthcare organizations in the US, Europe and Asia. Over 75 commercial programs with healthcare systems. Pharma partnerships with GSK, BI, Novartis, Orion and 3 unannounced. Acquired by ResMed in 2018 for $225 million.
3. Biofourmis
It was founded five years ago. The Boston-based startup uses predictive analytics to forecast problems in patients in heart failure, and is now looking to expand its work to other specialties.
The company’s analytics engine received FDA 510(k) clearance last fall. Shortly after, Biofourmis acquired Biovotion, a startup that makes an armband that collect data on heart rate, skin temperature, and a plethora of other vital signs.
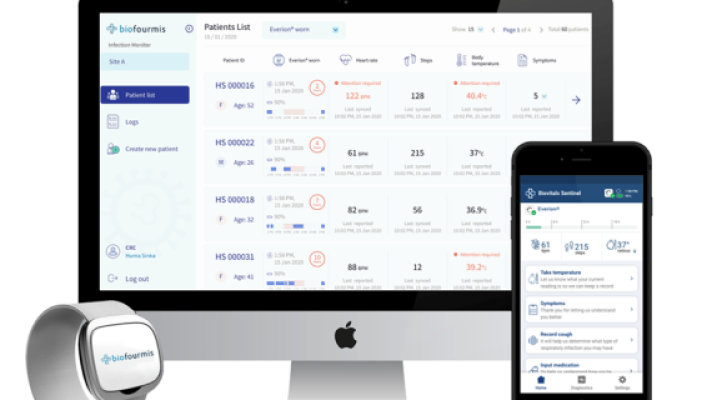
Biofourmis’ most recent addition is Gaido Health, a remote monitoring system for oncology patients. The company acquired Gaido Heath from Takeda Pharmaceuticals
With its new acquisition, Biofourmis plans to use Gaido Health’s platform to manage toxicities in cancer patients undergoing CAR-T cell therapy. Patients are normally monitored for at least a week after receiving treatment. With the acquisition of Gaido Health, Biofourmis hopes to allow patients to be monitored at home to detect early signs of clinical complications after receiving treatment.

Biovotion’s Everion device, worn on the upper arm, measures 22 different parameters in real time, including heart rate, respiratory rate, blood oxygenation, skin temperature and activity.
Biofourmis has been tapped by Novartis to help develop digital therapeutic programs that follow patients home after they’ve been recently diagnosed with heart failure.
Everion® is CE medical class 2a approvedas well as FDA 510(k) exempt listed as a medical device.
BiovitalsHF™V1: Prescription software to enable early detection of HF exacerbation and augment guideline-directed use of HF Medications
In May 2019, the Biofourmis won FDA approval for its Cloud-based Biovitals Rhythm Analytics platform that automates the interpretation of over 15 types of cardiac arrhythmias.
Two Recent Digital – Biopharma Deals
1. SHIONOGI & AKILI
Akili Interactive is a digital medicine company creating prescription treatments for people living with cognitive dysfunction and brain-related conditions.
Shionogi & Co., Ltd. is a major research-driven pharmaceutical company focused in two therapeutic areas: infectious diseases, and pain/CNS disorders.
AKL-T01 is currently under review with the FDA as a potential digital treatment for children with ADHD.
AKL-T02 is in late stage development as a potential treatment for cognitive dysfunction and related symptoms in children with Autism Spectrum Disorder. Shionogi will oversee clinical development, sales and marketing for both products in Japan and Taiwan.
2. OTSUKA & CLICK
Click Therapeutics, Inc. develops and commercializes software as prescription medical treatments through cognitive and neurobehavioral mechanisms.
Otsuka is a global healthcare company with a significant presence in the area of mental health. Otsuka has agreed to commit capital to fully fund development of Click’s novel mobile application CT-152 for Major Depressive Disorder, and to commercialize this application world-wide upon achievement of regulatory approvals.
CT-152 is a software application (app) that will leverage evidence-based cognitive therapy principles and Click’s patient engagement platform to treat patients either independently or in conjunction with prescribed pharmacotherapies; the app will be classified as Software as a Medical Device (SaMD). Otsuka will pay Click up to $10 million in upfront and regulatory milestone payments, along with an estimated $20 million in development funding.


Roadblocks and Challenges for Digital Therapeutics
Like with any other new technology, digital therapeutics will have to overcome major roadblocks before wide scale commercial adoption. Some major impediments are described below:
Regulatory challenges – Shorter developmental cycles aided through iterative processes means that the software updates will get rolled out very frequently. Monitoring & regulating such periodic updates will be very difficult for healthcare regulatory bodies. Hence, there is a need for regulatory bodies to develop new approval mechanisms to keep up with the pace of development. In fact, FDA has already launched a pre-certification pilot to explore new means of addressing the problem.
Payer adoption challenges – Sponsorship of payer organizations will be very crucial for the success of DTx solutions. Most of the solutions are aimed at treating chronic diseases which are prevalent for years. Hence, proving efficacy of these solutions will be extremely difficult. The success of these solutions will heavily rely on novel ways of real-world evidence collection.
Physician adoption challenges – Mainstream adoption of DTx solutions will have a great learning curve for the physicians. Since these solutions will completely alter the traditional care pathways, physicians will have to undergo several trainings before they could optimally use the solutions. A physician-centric design to reduce the burden of participation will go a long way to ensure meaningful adoption.
Future and Way Forward for healthcare organizations of delivering Digital Therapeutics
The global digital therapeutics market is expected to reach USD 457.9 Million by 2021 from USD 110 Million in 2016, growing at a CAGR of 27.7% from 2016 to 2021.” Markets & Markets
While today digital appears to only offer minor incremental improvements, it has the potential to truly create a paradigm shift in the biopharma sector
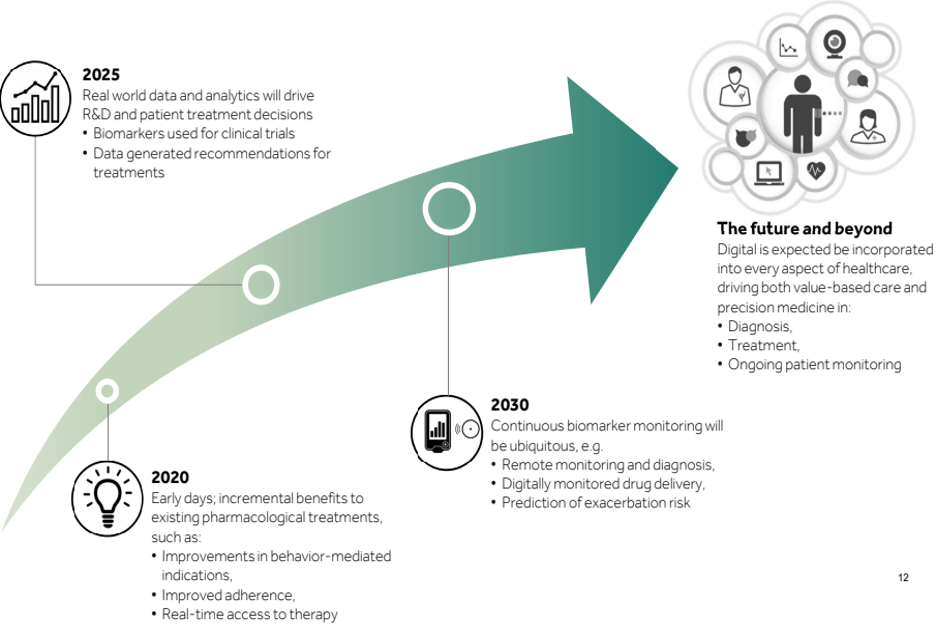
Healthcare companies lack the technical know-how of delivering digital therapeutic solutions. It requires a user-centric design thinking aided with iterative development cycles.
Partnering with a healthcare product engineering company that understand such technologies and works with an end-user orientation is the best way to move forward in such an uncertain environment. Here’s how enterprises should gear up to solve this digital challenge:
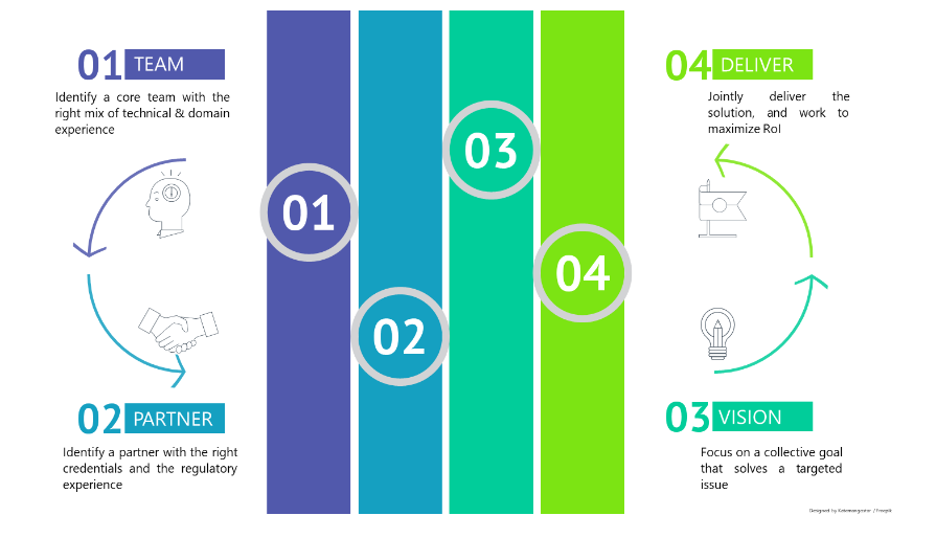
Creating Competency
Mapping internal champions who are adept and aware of the technologies, and a detailed understanding of what would be needed to be outsourced, will be the first step in re-imagining the organization’s capabilities and paving the path for a better healthcare foundation.
Finding the right partner(s)
Competency can be inherent or acquired. Finding the right partners who share a similar vision of growth and who maps with your values to complement your offering – be it technology, content or device often results in a win-win scenario
Focus Together
Once the right partner is identified, it is important to ensure both focus on a collective goal that solves a targeted issue and in turn enhances the competence and value of the organization with the collaborative effort.
Deliver
Closely work with the partners to deliver a user-centric solution with a faster time to market. Jointly work towards increasing market uptake and maximize the return on investments.
Conclusion
DTx includes software-based therapeutic interventions delivered by a digital interface and has the potential to affect the healthcare landscape.
The advancement of DTx will create many opportunities for healthcare professionals, and lead to innovative convergence of medicines, devices, and software.
All DTx products must be optimally exposed to ensure efficacy and safety when used as therapeutically indicated. While the term Digital Therapeutic may still be unfamiliar to many, these therapies are here to stay.
Digital Therapeutics (DTx) are becoming a new category of medicine, poised to address chronic and other hard to treat conditions.
While much work remains for digital therapies to be integrated into and across the traditional healthcare ecosystem, Digital Therapeutics will “increasingly influence the way healthcare is delivered and consumed across the world”.
References
*Chung JY. Digital therapeutics and clinical pharmacology. Translational and Clinical Pharmacology. 2019; 27(1):6-11.
*Sverdlov O, Van Dam J, Hannesdottir K, Thornton‐Wells T. Digital therapeutics: an integral component of digital innovation in drug development. Clinical Pharmacology & Therapeutics. 2018; 104(1):72-80.
*Dang A, Arora D, Rane P. Role of digital therapeutics and the changing future of healthcare. Journal of Family Medicine and Primary Care. 2020; 9(5):2207.
* Capobianco E. On digital therapeutics. Frontiers in Digital Humanities. 2015 10; 2:6.
* Guthrie NL, Berman MA, Edwards KL, Appelbaum KJ, Dey S, Carpenter J, Eisenberg DM, Katz DL. Achieving rapid blood pressure control with digital therapeutics: retrospective cohort and machine learning study. JMIR cardio. 2019; 3(1):e13030.
* Choi MJ, Kim H, Nah HW, Kang DW. Digital therapeutics: emerging new therapy for neurologic deficits after stroke. Journal of stroke. 2019; 21(3):242.
* Metcalf CS, Huntsman M, Garcia G, Kochanski AK, Chikinda M, Watanabe E, Underwood T, Vanegas F, Smith MD, White HS, Bulaj G. Music-Enhanced Analgesia and Antiseizure Activities in Animal Models of Pain and Epilepsy: Toward Preclinical Studies Supporting Development of Digital Therapeutics and Their Combinations With Pharmaceutical Drugs. Frontiers in neurology. 2019; 10:277.
* Luderer HF, Coolidge K, Campbell AN, Nunes EV, Maricich YA. reSET Digital Therapeutic for SUD Demonstrates Dose-Dependent Improvement in Outcomes. InJOURNAL OF PHARMACOKINETICS AND PHARMACODYNAMICS 2018 (Vol. 45, pp. S84-S85). 233 SPRING ST, NEW YORK, NY 10013 USA: SPRINGER/PLENUM PUBLISHERS.
* Burrone V, Graham L. Digital Therapeutics Past Trends and Future Prospects. Digital Technologies. 2020:32.
* Kim C. How Will the Digital Tools Change Healthcare?. Journal of Sleep Medicine. 2019; 16(2):71-4.


Samyak Nag and Shivani Baliyan M. Pharma students at Amity University Noida.
