When a new cancer drug shows promise, it trends globally. When a gene therapy corrects a rare mutation, headlines follow. But when a novel antibiotic advances against drug-resistant pathogens — silence.
That silence is dangerous.
The World Health Organization has repeatedly warned that antimicrobial resistance (AMR) is among the top global public health threats. Resistant infections already contribute to millions of deaths annually, with Gram-negative pathogens posing particular danger in hospital settings. Yet antibiotic innovation rarely receives sustained global celebration — or even serious public conversation.

The Visibility Gap
Consider Zaynich (WCK 5222), developed by Wockhardt. It combines cefepime with zidebactam, a β-lactam enhancer designed to overcome some of the most formidable resistance mechanisms in Gram-negative bacteria.
It has received Fast Track designation from the U.S. Food and Drug Administration — recognition reserved for drugs addressing unmet medical needs.
Scientifically, that’s significant. Media-wise? Muted. Why does this happen?
- Antibiotics Don’t Fit the Blockbuster Narrative
Antibiotics are short-course therapies. They are:
• Restricted by stewardship programs
• Used in hospitals, not retail markets
• Designed to be conserved, not consumed widely
Contrast that with oncology or chronic disease therapies that generate recurring revenue and long-term treatment arcs. Media ecosystems — especially business media — naturally gravitate toward commercial magnitude.
Antibiotics are built to save lives, not dominate quarterly earnings calls.

- The Shadow of Commercial Failures
The antibiotic sector has a sobering recent history. Companies like Achaogen gained regulatory approval for novel antibiotics, only to collapse financially.
That episode created a narrative of economic fragility around antibiotic R&D. Investors grew cautious. Editors followed suit.
So even when genuine scientific progress occurs, coverage tends to wait for “proof of durability.”

- Geography Shapes Global Attention
Breakthroughs from multinational giants headquartered in the U.S. or Europe often receive amplified coverage. When innovation emerges from outside traditional pharma power centers — even if it clears U.S. regulatory review — the narrative framing shifts subtly.
Instead of “global scientific milestone,” it becomes “regional industry success.”
That lens needs recalibration.
Antimicrobial resistance does not recognize geography. Neither should recognition of solutions.

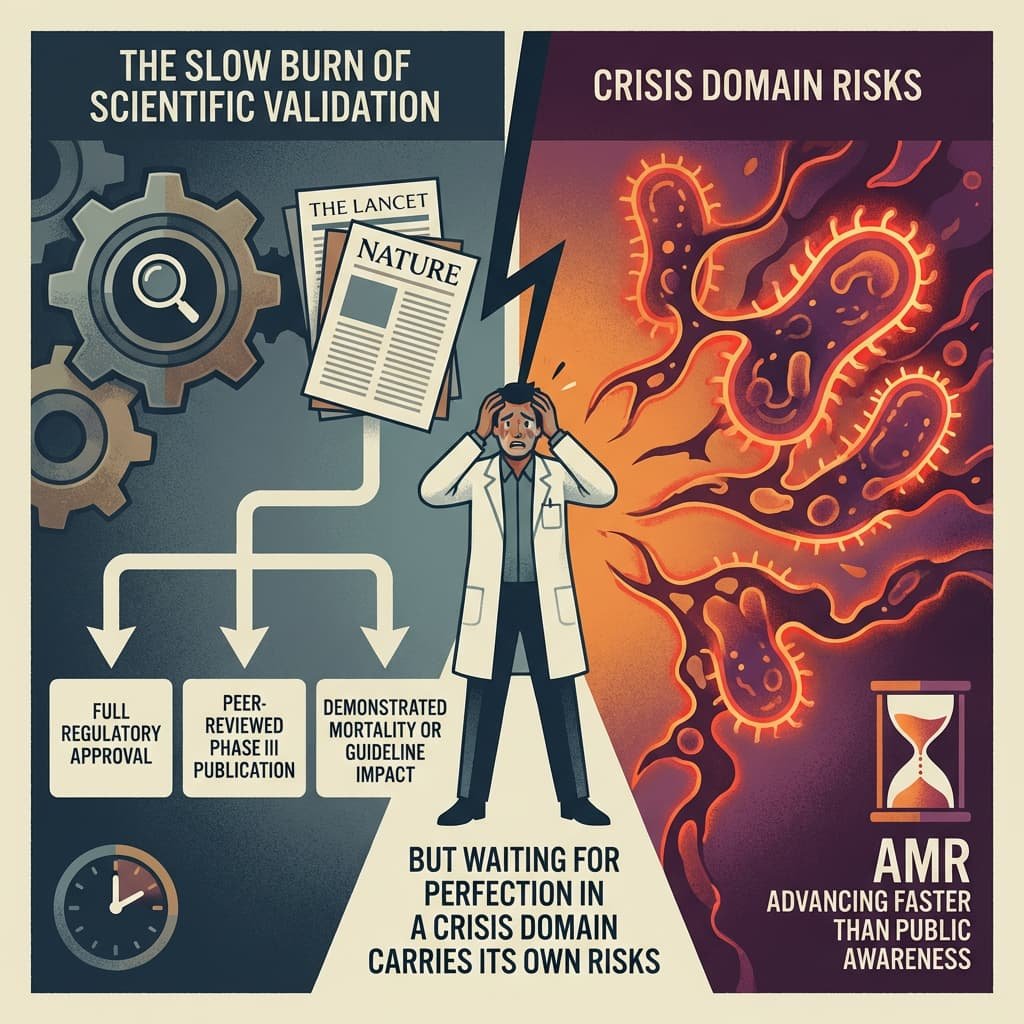
- The Slow Burn of Scientific Validation
Top-tier journals such as The Lancet and Nature typically amplify breakthroughs after:
• Full regulatory approval
• Peer-reviewed Phase III publication
• Demonstrated mortality or guideline impact
Until then, coverage remains largely within industry, financial, or specialist domains.
But waiting for perfection in a crisis domain carries its own risks. AMR is advancing faster than public awareness.
- The Real Stakes
Antimicrobial resistance is projected to reshape global mortality patterns if unchecked. The WHO’s priority pathogen lists underscore how thin the pipeline remains for novel Gram-negative agents.
Every viable addition to the antibiotic arsenal represents:
• Years of high-risk research
• Substantial capital commitment
• Scientific persistence in a neglected space
These are not incremental steps. They are strategic defenses against a systemic threat.
And they deserve proportional recognition.

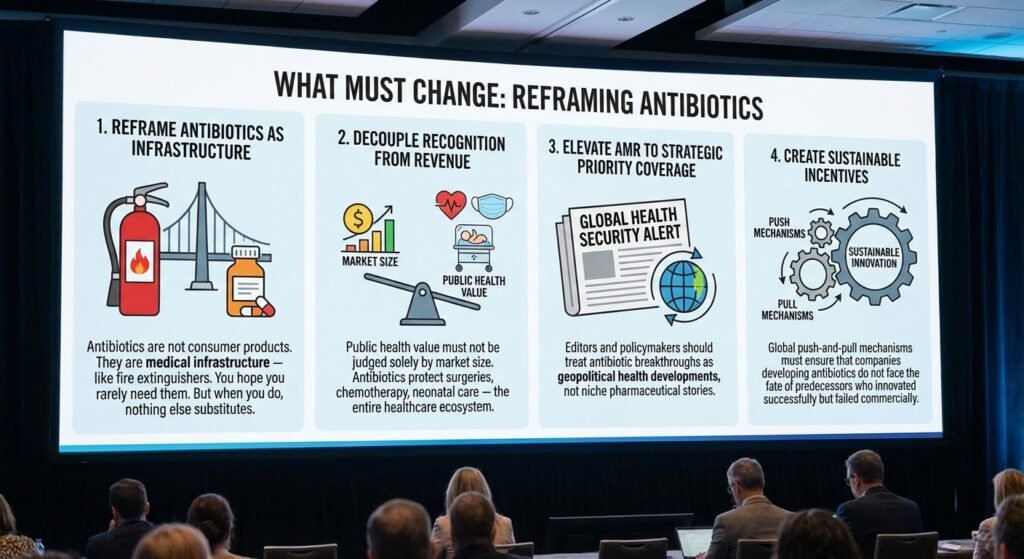
What Must Change
1. Reframe Antibiotics as Infrastructure
Antibiotics are not consumer products. They are medical infrastructure — like fire extinguishers. You hope you rarely need them. But when you do, nothing else substitutes.
2. Decouple Recognition from Revenue
Public health value must not be judged solely by market size. Antibiotics protect surgeries, chemotherapy, neonatal care — the entire healthcare ecosystem.
3. Elevate AMR to Strategic Priority Coverage
Editors and policymakers should treat antibiotic breakthroughs as geopolitical health developments, not niche pharmaceutical stories.
4. Create Sustainable Incentives
Global push-and-pull mechanisms must ensure that companies developing antibiotics do not face the fate of predecessors who innovated successfully but failed commercially.
The Bottom Line
We celebrate what we see as transformative. Antibiotic innovation is transformative — just less theatrical.
If the world continues to under-recognize breakthroughs in AMR, the message to innovators becomes clear: your risk is invisible. That is not a signal we can afford to send.
Because when resistance rises — and it will — the headlines will not ask whether we celebrated antibiotic breakthroughs. They will ask why we didn’t invest in them sooner.

Sources
1. World Health Organization — Global reports on antimicrobial resistance burden and priority pathogens.
2. U.S. Food and Drug Administration — Fast Track designation framework and antimicrobial development guidance.
3. Wockhardt — Public disclosures and development updates on WCK 5222 (Zaynich).
4. Achaogen — Case study of antibiotic developer insolvency post-approval.
5. The Lancet — Published research on global AMR burden.
6. Nature — Commentary and analysis on antibiotic innovation pipeline challenges.

All Images are AI Generated for Illustration Only. E&OE