In India, where urban sleep debt is rising, obesity rates are increasing, and CPAP adoption remains minimal, a pill could theoretically transform access.

A decades-old therapeutic stalemate in sleep medicine is poised to break. For the first time, a pharmaceutical treatment for obstructive sleep apnea (OSA) is approaching the U.S. market.
Apnimed, a Boston-based clinical-stage company valued at approximately $400 million, is preparing to submit its lead candidate, AD109, for approval with the U.S. Food and Drug Administration. If successful, the oral therapy would become the first pharmacologic alternative to the Continuous Positive Airway Pressure (CPAP) machine—a device that has remained the standard of care for nearly four decades despite well-documented adherence challenges.
The announcement, featured in a recent Forbes profile, signals what industry observers describe as a potential inflection point for a condition affecting an estimated 80 million Americans and nearly one billion adults worldwide.

The Adherence Problem: Why CPAP Alone Has Not Been Enough
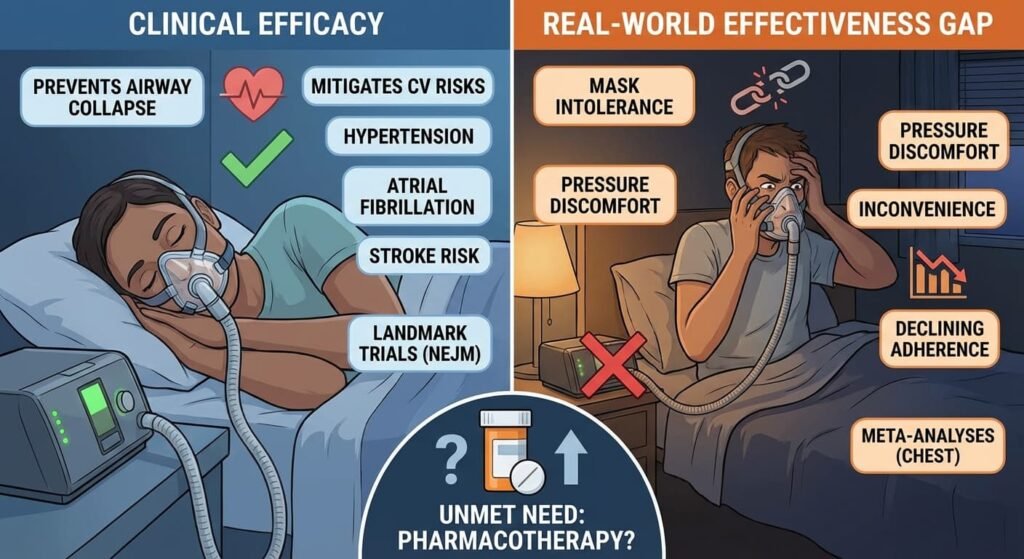
The clinical efficacy of CPAP is not in question. When used consistently, it effectively prevents airway collapse and mitigates the cardiovascular consequences of OSA, including hypertension, atrial fibrillation, and stroke risk. Landmark trials published in the New England Journal of Medicine have established this link.
However, real-world effectiveness has lagged behind clinical efficacy. Meta-analyses in journals such as Chest consistently report that a substantial proportion of patients cannot tolerate the mask, the airflow pressure, or the inconvenience of the device. Adherence rates decline sharply within the first months of prescription.
This gap between efficacy and adherence has created a persistent unmet need—one that pharmacotherapy could potentially address.
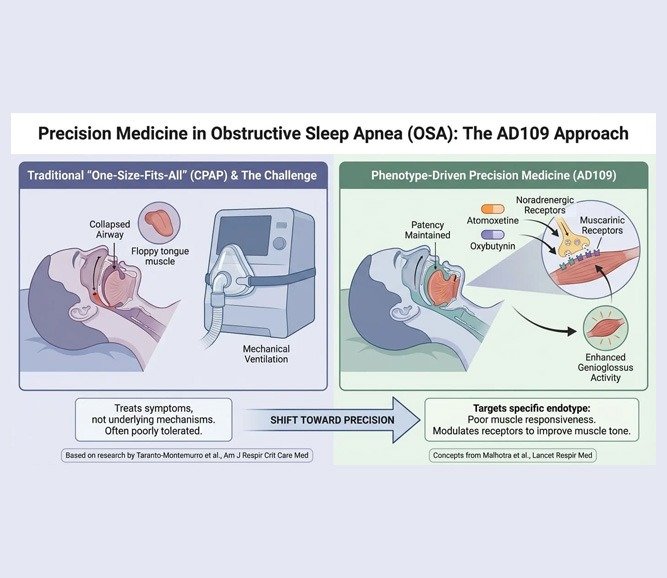
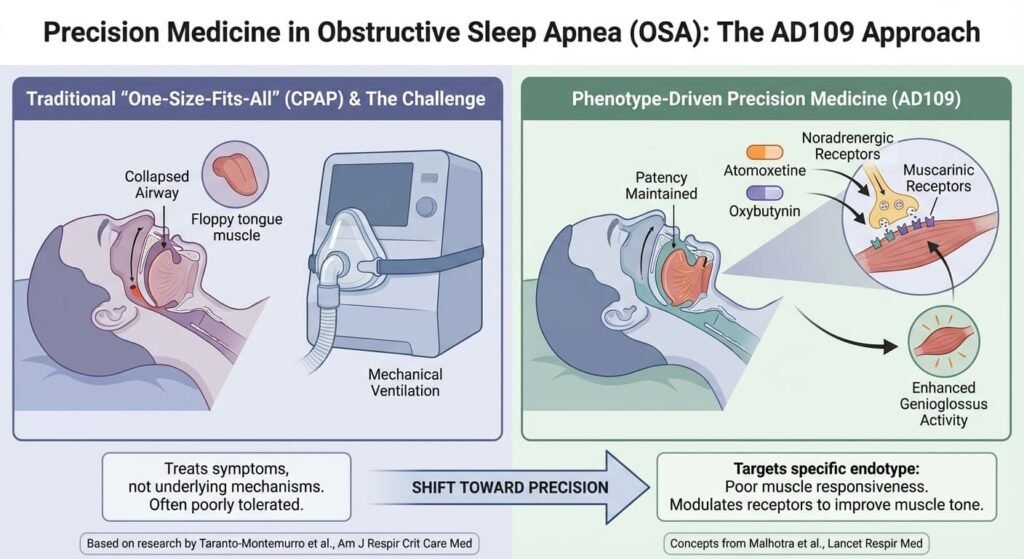
The Science Behind AD109: Targeting Airway Muscle Tone
AD109 is not a sedative, nor does it target the respiratory drive. Instead, it is a combination therapy (atomoxetine and oxybutynin) designed to address a specific physiological phenotype of OSA: poor upper airway muscle responsiveness during sleep.
The scientific foundation for this approach was established by Taranto-Montemurro and colleagues in the American Journal of Respiratory and Critical Care Medicine. Their research demonstrated that by modulating noradrenergic and muscarinic receptors, the combination could enhance genioglossus (tongue muscle) activity, thereby maintaining airway patency without disrupting sleep architecture.
This represents a shift from a “one-size-fits-all” mechanical approach toward phenotype-driven precision medicine. As Malhotra et al. outlined in The Lancet Respiratory Medicine, OSA is not a single disease but a syndrome with multiple underlying mechanisms. Stratifying patients by endotype—poor muscle tone, low respiratory arousal threshold, high loop gain—allows for targeted pharmacologic intervention.
The GLP-1 Connection: A Converging Therapeutic Landscape
Apnimed’s candidate is not the only pharmaceutical approach under investigation. The emergence of highly effective GLP-1 receptor agonists for obesity management has opened a parallel pathway for OSA treatment.
Given that excess adiposity—particularly around the upper airway and abdomen—is a primary risk factor for OSA, drugs such as semaglutide (Wegovy) and tirzepatide (Mounjaro/Zepbound) are being actively studied for their effects on sleep-disordered breathing.

Eli Lilly’s dedicated Phase 3 program, SURMOUNT-OSA, is expected to provide pivotal data on whether significant weight reduction translates into clinically meaningful improvements in the Apnea-Hypopnea Index (AHI). Early evidence from the STEP-1 trial suggested that semaglutide users experienced reductions in OSA severity proportional to weight loss.
The future therapeutic landscape may therefore involve stratification: GLP-1s for the obesity-driven phenotype, and neuro-modulatory agents like AD109 for patients with primary neuromuscular vulnerability. Combination strategies are also plausible, addressing both the root cause and the immediate mechanical collapse.
The Regulatory Hurdle: Cardiovascular Outcomes
For any new therapy to achieve broad adoption—and reimbursement—it must demonstrate more than an improvement in the AHI. The FDA and payers will require evidence of cardiovascular benefit or, at minimum, non-inferiority to CPAP in reducing major adverse cardiovascular events (MACE).
CPAP has outcome data. A pill must either match it in the general population or demonstrate clear value in the specific subset of patients who cannot tolerate the device. Without such data, a drug risks being positioned as a second-line adjunct rather than a first-line alternative.

Implications for Global Access: The Indian Context
The potential introduction of an oral therapy carries particular significance for markets with low diagnostic and treatment penetration. In India, where urban sleep debt is rising, obesity rates are increasing, and CPAP adoption remains minimal, a pill could theoretically transform access.
However, as the original Forbes analysis noted, pricing strategy will determine whether this becomes a mass-market therapy or a niche metro product. Affordability constraints in price-sensitive markets will require manufacturers to make deliberate choices about access and tiered pricing.

Conclusion: A Therapeutic Category at Inflection
The filing of AD109 with the FDA represents the culmination of decades of research into the pathophysiology of sleep apnea. It also opens a new chapter in which sleep disorders may receive the same innovation intensity that has transformed the treatment of diabetes and obesity.
Whether this particular candidate succeeds or fails, the direction of travel is clear. Sleep is no longer a lifestyle conversation. It is a cardiovascular, metabolic, and commercial frontier.
As the Forbes headline suggests, the “holy grail” may be nearing market entry. But even if AD109 clears the regulatory bar, the broader transformation of sleep medicine will depend on a portfolio of approaches—neuro-modulatory drugs, metabolic therapies, and potentially their combination—to address the heterogeneous nature of the disease.
For the 80 million Americans and nearly one billion people worldwide living with sleep apnea, the era of “CPAP or nothing” is finally coming to an end.

All Images are AI Generated for Illustration Only. E&OE
This is a Fully Media-TED MedicinMan Medical Perspective – Listening to, and Amplifying Healthcare Voices
Appendix: Key Scientific and Clinical References
- Taranto-Montemurro, L., et al. (2019). The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity: A Randomized, Placebo-controlled, Double-Blind Crossover Trial. American Journal of Respiratory and Critical Care Medicine, 199(10), 1267–1276.
· Phase 2b proof-of-concept study for AD109. - Malhotra, A., et al. (2021). Endotypes and phenotypes in obstructive sleep apnoea. The Lancet Respiratory Medicine, 9(5), 439–440.
· Framework for phenotype-driven precision medicine in OSA. - Weaver, T. E., & Grunstein, R. R. (2008). Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proceedings of the American Thoracic Society, 5(2), 173–178. (Data consistently cited in Chest).
· Evidence base for CPAP adherence challenges. - McEvoy, R. D., et al. (2016). CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. New England Journal of Medicine, 375(10), 919–931.
· Landmark trial establishing cardiovascular outcomes with CPAP. - Benjafield, A. V., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet Respiratory Medicine, 7(8), 687–698.
· Definitive global prevalence study. - Wilding, J. P. H., et al. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine, 384(11), 989–1002. (STEP-1 trial, OSA sub-analysis).
· Evidence for GLP-1 effects on OSA severity. - ClinicalTrials.gov. (2023). A Study of Tirzepatide (LY3298176) in Participants With Obesity and Obstructive Sleep Apnea (SURMOUNT-OSA). NCT05412004.
· Ongoing Phase 3 trial for tirzepatide in OSA. - Forbes. (2025). The ‘Holy Grail’ Sleep Apnea Pill Could Be On The Market Next Year. Forbes Magazine.
· Primary source for Apnimed’s FDA filing preparation.