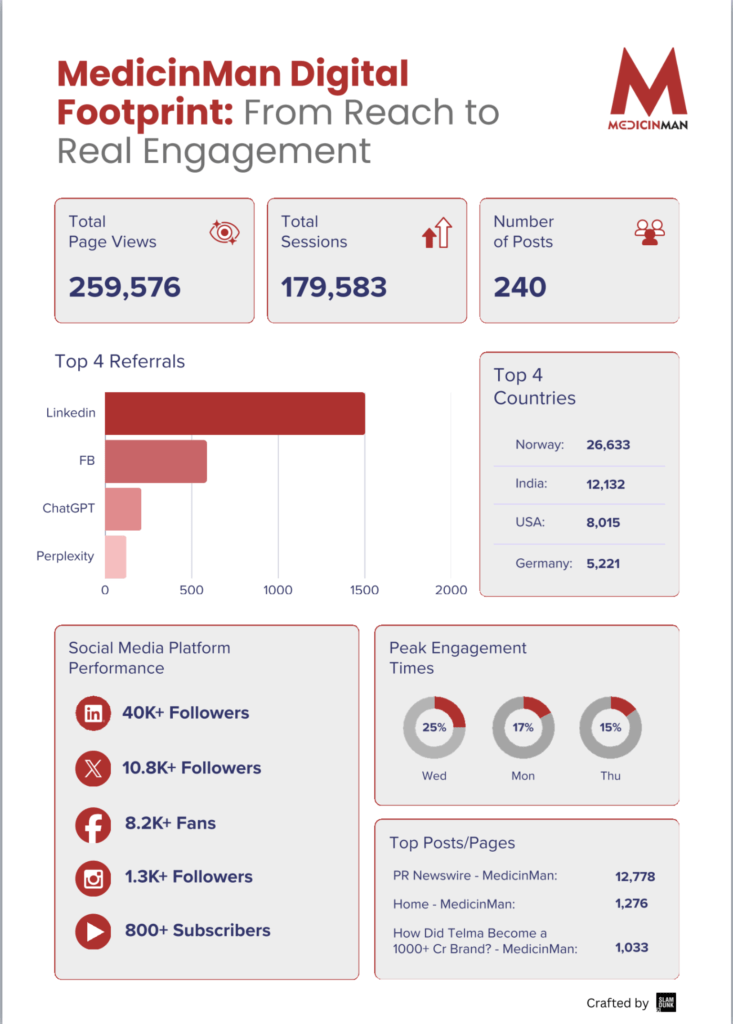
The Telangana Drugs Control Administration’s stop-use notice on a paediatric syrup contaminated with ethylene glycol should not be framed as an isolated quality lapse. It is better understood as a stress test the system failed — again.
Ethylene glycol contamination is not novel, obscure, or technically hard to detect. Its toxicology is well known. Its detection is routine. When it appears in a medicine meant for children, the failure is not scientific — it is regulatory.

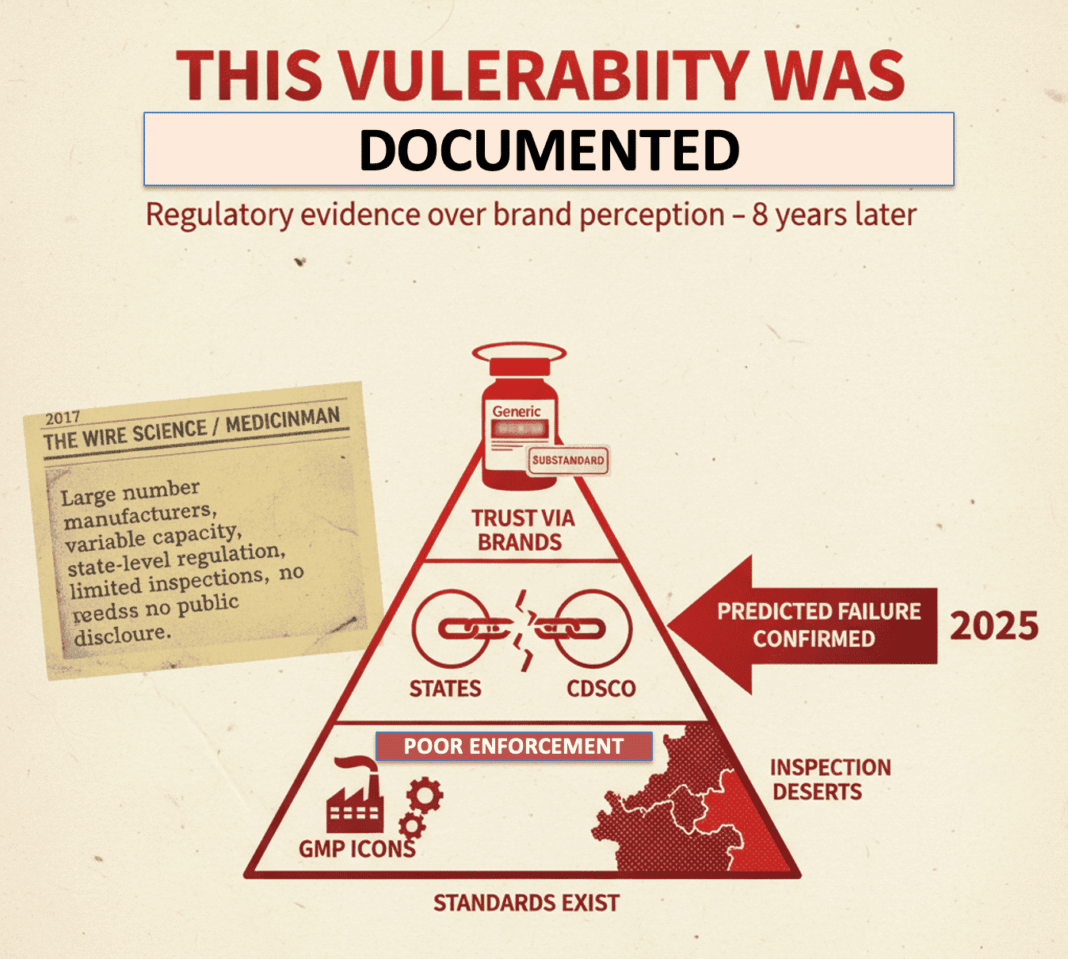
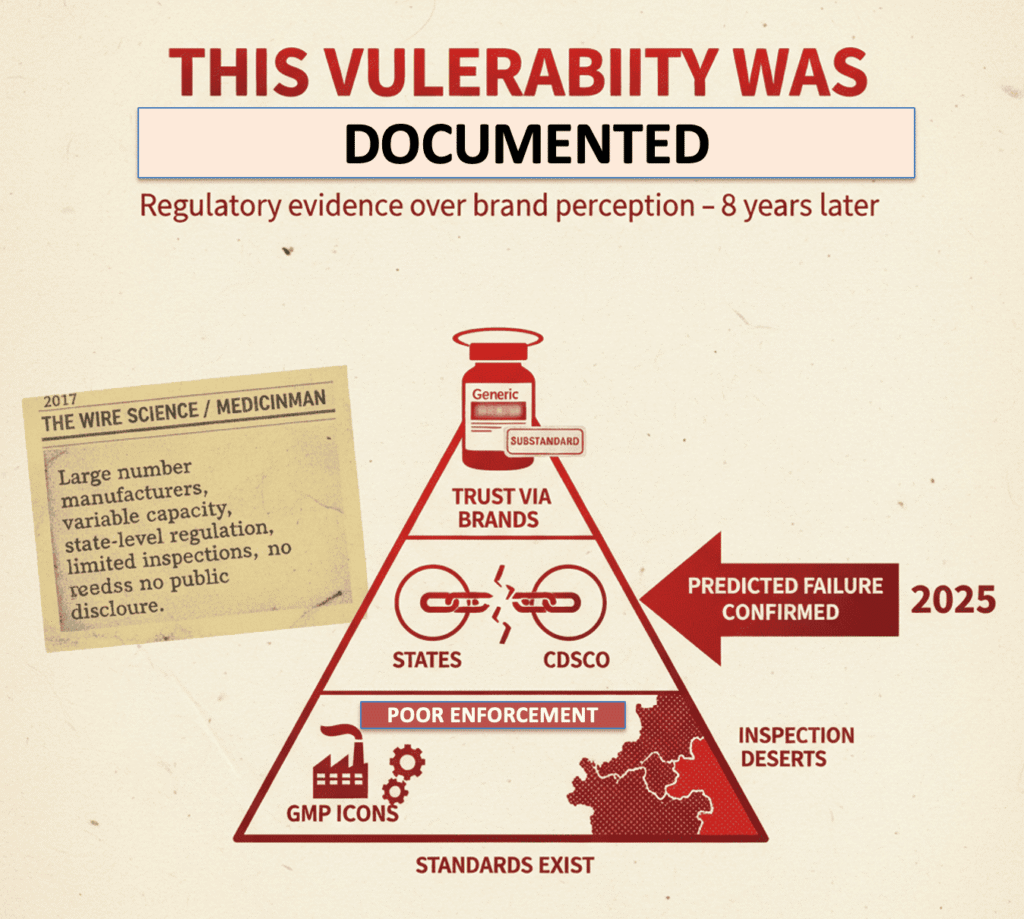
This Vulnerability Was Documented Years Ago
In 2017, an article published in The Wire Science and later in MedicinMan laid out a structural concern in India’s drug ecosystem:
India’s pharmaceutical market is characterised by a very large number of manufacturers with highly variable technical capacity, regulated primarily at the state level, with limited inspection bandwidth and minimal public disclosure of failures.
The argument was straightforward and empirical:
• regulatory standards exist,
• enforcement is uneven,
• and trust is often outsourced to brand perception rather than regulatory evidence.
The Telangana incident aligns precisely with that diagnosis.
Why the “Generics Are Equal” Debate Misses the Point
Recent claims that Indian generics are broadly equivalent in quality — based on limited batch testing — have been widely circulated. The data presented show that some sampled medicines met pharmacopoeial specifications.
That finding is not irrelevant. But it is insufficient.
As Dinesh S. Thakur and Prashant Reddy have consistently argued, including in response to similar claims:
• Pharmacopoeial compliance is a baseline, not a guarantee.
• Until recently, bioequivalence testing was not uniformly mandatory for generics in India.
• Point-in-time audits cannot replace continuous, regulator-led surveillance.
A medicine can pass chemical tests and still fail therapeutically. More importantly, systemic risk is not disproved by selective compliance.
What the Telangana Case Actually Reveals
Ethylene glycol contamination indicates breakdowns across the value chain:
• raw material qualification,
• solvent sourcing,
• batch testing,
• inspector oversight,
• and recall speed.
None of these steps requires new science or new laws. They require capacity, consistency, and consequence.
The issue is not that India lacks regulations.
It is that regulatory depth thins out dramatically at the lower end of the manufacturing spectrum — precisely where margins are tight, oversight is sporadic, and detection is often reactive.

The Real Question (Still Unanswered)
The debate should not be framed as branded vs generic or cheap vs expensive.
The operational question is simpler and more uncomfortable:
How often are small and mid-scale manufacturers inspected, how rigorous are those inspections, and how transparently are failures disclosed to the public?
Until that data is routinely published, every contamination episode will be treated as a surprise — despite being structurally predictable.

One Policy Ask (No Grandstanding)
If there is one reform that would materially reduce risk, it is this:
Mandatory public disclosure of inspection frequency, major violations, and test failures — manufacturer-wise, not incident-wise.
Not press releases after harm occurs.
Not reassurances.
Just data.
Transparency is not punitive. It is corrective.

Bottom Line
The vulnerabilities identified in 2017 remain intact in 2026.
The Telangana syrup case does not introduce a new problem — it confirms an old one.
India does not suffer from a generic drug problem.
It suffers from a regulatory visibility problem, most acute among its smallest manufacturers.
Until that is fixed, quality failures will continue to surface not in audit reports, but in emergency advisories — after exposure has already occurred.
That is not a failure of intent.
It is a failure of governance.

Sources:
1. Telangana Drugs Control Administration (TGDCA), Stop-Use Notice for Almont-Kid Syrup following CDSCO laboratory alert, 2026.
2. The News Minute, “Coldrif Cough Syrup Deaths: Tamil Nadu Cancels Sresan Pharma Licence,” 2025.
3. The Week, “Sresan Pharma Owner Arrested After Toxic Syrup Deaths,” 2025.
4. Times of India, “Drug Inspectors Suspended After Cough Syrup Linked to Child Deaths,” 2025.
5. NDTV, “Explainer: How Toxic Cough Syrup Passed Regulatory Checks,” 2025.
6. India Today Health, “Are Generic Medicines Inferior? What the Data Shows,” 2024.
7. Soans, Anup. “Quality, Not Price, Is the Key Issue When Prescribing Generic Drugs in India.” The Wire Science, 2017.
8. Economic and Political Weekly. “The Pharmaceutical Industry Has Vested Interests in Making Arguments against Generic Drugs,” citing Soans (2017).
9. Thakur, D.S. & Reddy, P. The Truth Pill: The Myth of Drug Regulation in India. Juggernaut, 2022.