
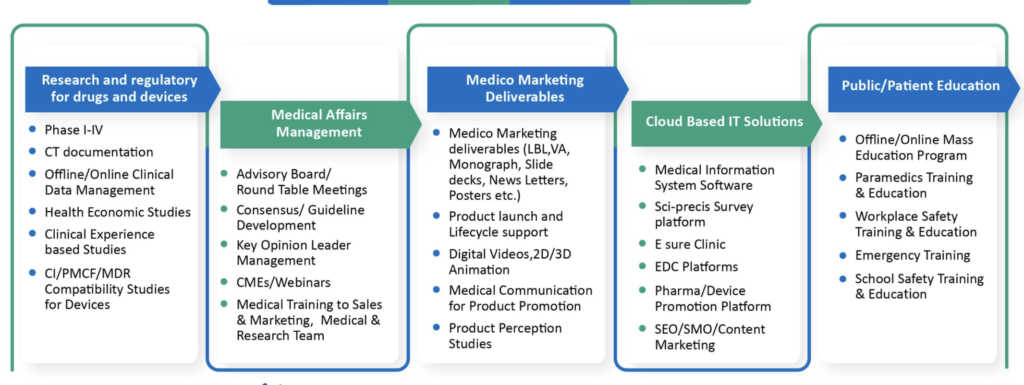
The Medical department comprising of Clinical Operations (the ones who generate data), Regulatory Affairs (the ones who submit data to the regulator for approval), Medical Affairs (the ones who bring out the value of that data for the customer), Pharmacovigilance (the ones who do benefit to risk evaluation of a product), and Quality Medicine (the ones who are in charge of audits, compliance and training) is an integral cog in the wheel of a biopharmaceutical company.
It is like an autorickshaw where the front wheel represents Sales while the two back wheels represent Marketing and Medical.

If all three functions work in sync with each other, if they are one with each other and one with the business, it is easier to become number one. It is important for Medical to remember that Sales and Marketing are their customers besides, of course, the external customers (doctors). When a medical manager joins a biopharmaceutical company s/he is first and foremost a business manager and needs to have a fair understanding of the business. For sure, s/he is not expected to do what a marketing or sales colleague does, and s/he is expected to use his/her medical expertise combined with business acumen to apply the learnings optimally for the business.
Just as how marketing is evaluated from a performance appraisal perspective, medical affairs personnel too need to be similarly evaluated. After all, both medical affairs and marketing need to jointly design and conceptualise the brand plan with medical and marketing elements, keeping the 4 Ds principles in mind, viz., Define, Design, Deliver and Dent (“make a dent in the universe” – Steve Jobs). The value of medical affairs to the business will automatically be brought out. One difference is the ratio of medical affairs to marketing and sometimes the medical manager has to serve the competing priority needs of many product managers which can be a challenge. The other difference is marketing (not medical affairs) does play a role in deciding targets for the field force. But since both medical affairs and marketing support sales colleagues, 50% of their evaluation can be based on how the product fares in the market place vis a vis pre-defined targets and 50% can be based on a qualitative and quantitative impact of their respective inputs on the business. Ideally they need to also sit together in brand team clusters.
The medical affairs professional is closest to sales and marketing but all the other four medical functions need to understand that they too need to be in sync with each other and with the business. It is like the five fingers of the hand when closed together become a formidable fist, all different, yet when these differences are for each other (diversity), it makes all the difference. And one need not break these silos, one needs to simply inter-connect them (‘syntegrated’ = synergy + integration). One needs to have the basic qualification for each of the five functions and some functional expertise is preferred. But one can also take freshers from academia (so that there is no need to unlearn and relearn) who can learn on the job and climb the learning curve quickly.
For a product physician s/he needs to know the basics and also needs to be continuously updated in the specific therapeutic area around the product, besides the product itself. A generalist is preferred (MD Pharmacology/Medicine) but as Subroto Bagchi has mentioned in his book, The Professional, “one may be professionally qualified but that need not necessarily qualify one to be a professional.” In other words, even an MBBS, PhD, M Pharm (Pharmacology), Pharm D could be very useful in a medical affairs team. One needs to know the subject matter in depth but one should also know how to communicate the same to the field force, marketing colleagues, and doctors (different strokes for different folks). When one trains the field force, one needs to “sell” the product to them, because if they are not sold on the product, how will they be able to sell it to their doctors? From this perspective, perhaps even medical affairs and marketing need to be trained on selling skills.

Perhaps the first few days could be spent in getting to know the role requirements, the Standard Operating Procedures (SOPs), the people within the medical team as well as in sales and marketing besides the other functions (induction, orientation, on-boarding), the product/s (from the classified index of medical information on the product), and working with the line manager, till one is judged to be able to manage independently. Essentially, a product physician is expected to be the product specialist knowing all that needs to be known and communicated on the product, its place in the guidelines and therapeutic armamentarium vis a vis other products in the standard of care, systematic reviews, regulatory documents, clinical trial data, safety profile, etc.
Since one will need to present the product to key opinion leader clinicians, who are experts in their field, one has to be able to rise to their expectations and quality standards to gain respect and credibility. This is where medical affairs can make a seminal difference, provided they know how to read the data, give meaning to it (information), and help the clinician in interpreting the evidence, and extrapolating it to their individual practice, so that they can help doctors match the right patient to the right drug. It is not always that one drug is better than another drug. More often it is about helping doctors identify the patient substrate that will respond to a given drug the best, in terms of safety and efficacy. Rather than always try and win over the customer or competitor, sometimes it is good to win the customer and competitor over. And sometimes one may also collaborate with competitors, grow the market, create entry barriers, and more patients benefit.
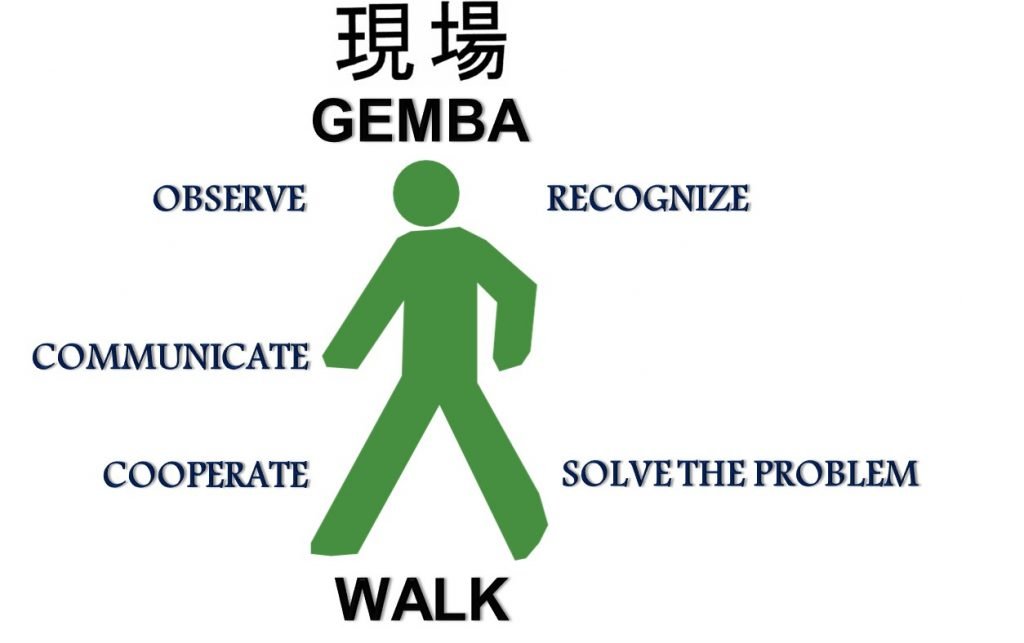
One can do preceptorships (go to the gemba) with the customer understanding his/her practice from his perspective and try to unite this customer insight with the positioning of the product promise, sometimes going beyond the pill with value-added services that help patients come to therapeutic goals and remain at therapeutic goals, so that they benefit and do not have complications due to uncontrolled disease.
Field-based medical affairs are specifically recruited for this medical relationship management but the same can be done to some extent by head office based medical affairs personnel too. This customer relationship management, exemplified best by sales colleagues, is also a domain expertise that medical needs to imbibe. Indian KOLs can be developed into global speakers and can be part of global advisory board meetings on design of clinical development plans of new products. In a highly cluttered and competitive environment, the real battle is not so much in the market place, as it is in the mind of the customer (Positioning – Al Ries, Jack Trout), where brand representatives need to be top of mind. For sure the product speaks for itself but it is also the conduct and the way the company colleagues carry themselves in front of the doctor that differentiates them from the others. They literally personify the product. If they manage to touch his/her “emotional” retina, and manage to sell the experience of being associated with the company and its products and services, they enhance their brand recall in his/her mind.
Since medical affairs is considered the data custodians they need to know how to identify unmet medical needs of the relevant market, and plug the gaps in local medical literature by facilitating the generation of primary data by the doctors. In addition to market research, the medical department can contribute by doing what is called as medical research, collecting epidemiology data (incidence, prevalence), which can sharpen the business forecasts/estimates. Guidelines released by medical societies can be updated regularly with scientific and financial help from the pharmaceutical industry. Practice-based real world evidence (vide infra) also has an important role to play to complement what is obtained from randomized controlled clinical trial data. Medical affairs can also do a lot with existing data, e.g., from electronic health records. They can partner with KOLs who are very good with systematic reviews (including meta-analyses). Publications that result from initiatives such as advisory board meetings or campaigns (such as Guidelines Applied to Practice or GAP programs that bridge the gap between what guidelines recommend and what doctors practice) can be followed by information dissemination programs that eventually drive improvement in outcomes.

New product planning (ideation and evaluation) is another area where medical, clinical and regulatory affairs colleagues can work closely with business development, portfolio, intellectual property professionals, marketing and sales to come up with business cases that decide which products need to be launched, as soon as possible, such that the value of these assets are realized per the plan.
One area where medical affairs personnel need to be more customer-facing is in presenting to doctors on and around the products. They do so at advisory board and advocacy board meetings but they can do so at customer launch meetings and continuing medical education meetings too, and can moderate international speaker programs too. One could have a clinico-pharmacological correlation where a senior clinician can present clinical aspects while the product physician presents on the product and answers questions. The entire medical affairs team can be recruited for certain blitzkrieg meetings around new product launches or CT result readouts, as they need to be fungible. This way one can have some meetings where KOLs address audiences but the rest of the must see list (MSL) of prescribers can be presented to by medical affairs personnel.
The current pandemic has made digital (digit = finger) interactions very critical in ensuring connect is maintained and one is able to “touch” the customer through this medium where content is king. Such meetings can be accreditated by state medical councils which help doctors in renewing their registration. This “tec(h)tonic” shift makes it very important for the medical representative (needs to represent what medical stands for, which is why medical needs to train the field force effectively) to also literally be the visual aid for the doctor, maintaining safe distance and yet able to communicate the take home message succinctly and consistently. Doctors can be connected by the field force to medical affairs colleagues in real time even as the detailing is happening so that queries are immediately resolved “face to face”. The iPad can also be made as animated and interactive (gamification, micro-learning) as possible to fully involve the doctor in a dialogue. Rather than only push information to doctors through the electronic medium, one should be able to create demand and pull for the doctor such that at any time the company is ready to connect with the doctor when s/he wants to do so.

Medical affairs are also into clinical development and need to work together with clinical operations (in-house or out-sourced) to design locally relevant clinical studies. In a pandemic it is imperative that one thinks of designing remote or digital or e-clinical trials where the patient need not come to sites and where the trial can go to the home of the patient. Technology [e.g., tele-consultation, wearable apps, real-time 24 hour ambulatory blood pressure or continuous glucose monitoring systems, Holter, sleep apnea monitors, mobile data (health in your hands)] can make some of it possible. If India is a part of global studies, then the Indian data not only helps expedite local approval, but it is also a source of competitive advantage as the investigators can also be spokespersons for the product, having had first-hand experience of the product, even before launch. Medical affairs can facilitate investigator initiated academic clinical research with the help of KOL investigators to plug gaps in the medical literature in the therapeutic area around the product, years before launch of a product. Naturally these KOLs can become spokespersons for the product at the time of launch. With the help of clinical operations, select Indian doctors can be trained and developed to be global investigators for future studies. Their centers can be developed into preferred research centers and core research sites. External medical affairs is another function where medical interacts with government (along with public/corporate affairs department), academia/universities/organizations such as the Indian Society for Clinical Research [including ethics committees and government medical colleges where Subject Expert Committee (SEC) members are generally affiliated], and medical professional societies, building trust.
Regulatory affairs can help shape regulatory policy by working closely with officials from the Central Drugs Standard Control Organization (CDSCO), and help expedite regulatory approval, thus advancing peak sales of a product. The locally approved prescribing information of a product (label) should be detailed by the medical representative and then the label can be left behind with the doctor (LBL = Leave Behind Label), with some documentation of the same, so that it informs the doctor about the product, and also protects the company in case the doctor uses a product off-label and lands up in a medico-legal case.
Pharmacovigilance (PV) helps the field force (FF) in managing doctors who report adverse events, trying to do a causality assessment, and in the process assuring the doctor of the continuing safety profile of the product, thus mitigating the potential loss of sales. When PV personnel do field work, they understand what the FF have to experience, and they also learn from the doctors, which helps them become more than just people who process adverse events. Together with medical information, PV is a source of market intelligence which can be put to good use for the respective brand teams (MIAMI = Medical Information As a source of Market Intelligence). MI can be a useful source of answering unsolicited medical information queries on off-label use of products. One can brand the MI function, promote it as a product, and even have it on the company website. Quality medicine is about ensuring all medical personnel are adequately trained on the SOPs and are well equipped for audits and inspections. They help conduct root cause and gap analysis and come up with Corrective And Preventative Actions (CAPAs) which can be useful even for business colleagues in their operations.
Medical affairs are also custodians of compliance with Legal and the Compliance teams on the way the company interacts with healthcare professionals in terms of finalizing the fair market value for deciding fee for service honoraria per applicable local regulatory and legal requirements. So while the medical, regulatory, legal and compliance teams may be viewed as the seat belt of the company, protecting it from harm, they do not come in the way of fast-paced growth. The best way of describing the value of medical is to say, “Know Medical, Know Business”. In other words, without a medical team it is indeed difficult, if not impossible, to do quality, compliant, ethical and value-based business. But it is also important for medical to know the business and, who knows, perhaps medically qualified personnel can also get degrees in pharmaceutical business management or an MBA, work in business functions in rotation, and eventually go on to become the CEO of the company.







