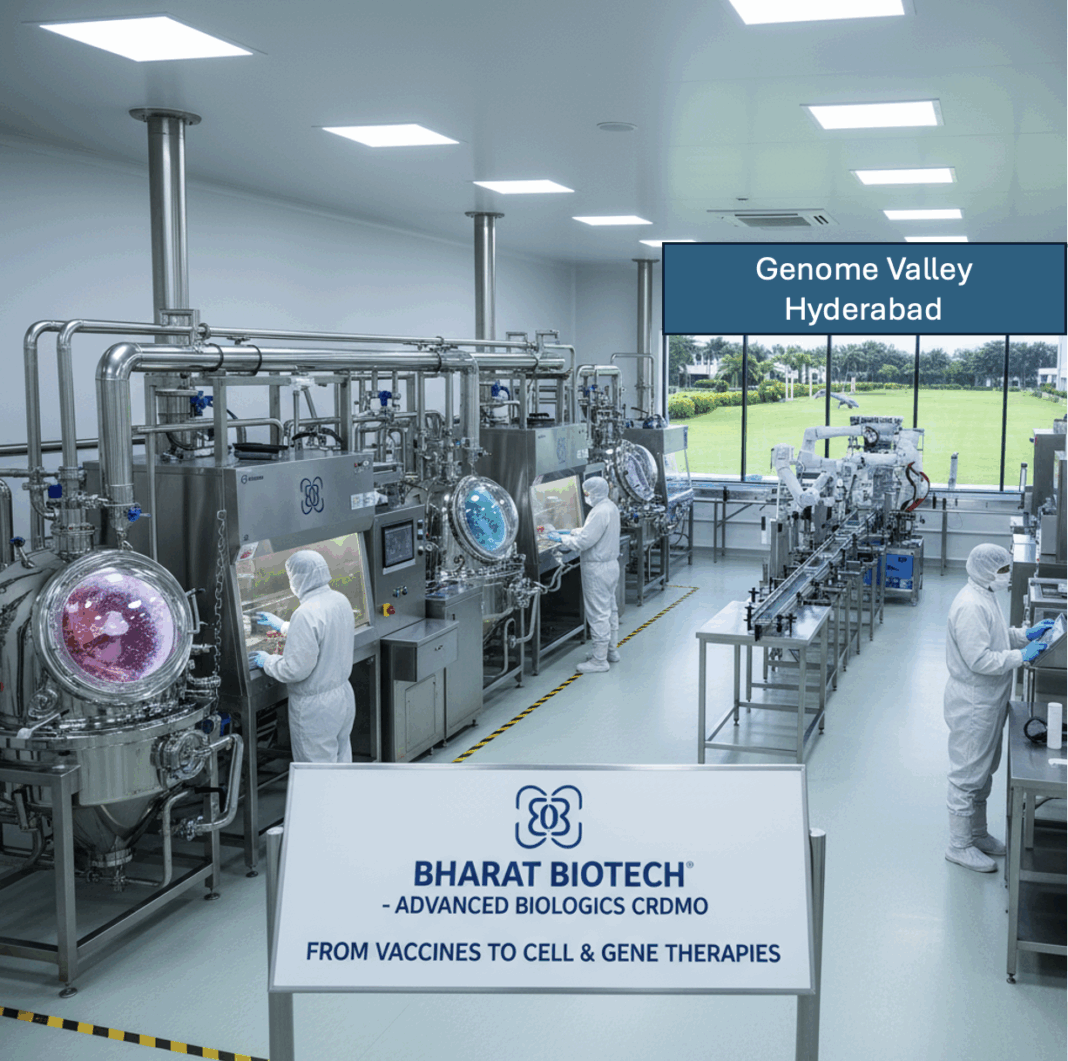
India’s pharmaceutical sector is at a tipping point. Long celebrated as the “pharmacy of the world,” the country is now pivoting from being a volume-driven generics powerhouse to becoming a hub for deep-tech, biology-based innovation. The launch of Nucelion Therapeutics Pvt Ltd, a wholly owned subsidiary of Bharat Biotech International Ltd. (BBIL), marks a significant milestone in this transition.
From Vaccines to Cell and Gene Therapies
Bharat Biotech, best known globally for its vaccine innovation—including Covaxin—has now entered the fast-evolving world of cell and gene therapy through its new Contract Research, Development and Manufacturing Organisation (CRDMO).
Located in Genome Valley, Hyderabad, the new 30,000 sq ft facility will specialize in the development and GMP manufacturing of plasmid DNA, viral and non-viral vectors, autologous and allogeneic cell therapies, and aseptic fill/finish operations. This is infrastructure of the kind that powers next-generation biologics worldwide.

The company’s therapeutic focus is on oncology, autoimmune diseases, and rare genetic disorders—areas at the forefront of global innovation where India has so far been a marginal player.
A Strategic Shift with Global Ambitions
This move isn’t just a business expansion; it’s a strategic reimagination of what Indian biotech can stand for. Nucelion has been designed to operate with independent governance, international compliance systems (FDA/EMA), and an arm’s-length relationship even with its parent company—clearly signaling intent to serve global innovators.
By entering the CRDMO space, Bharat Biotech is plugging a crucial gap in India’s biotech value chain: scalable, high-compliance manufacturing for emerging therapies. Until now, most startups and research institutions working on gene or cell therapy had to look abroad for process development and manufacturing support. Nucelion offers a local, globally benchmarked solution.
Why This Matters for Indian Pharma
For decades, India’s pharma growth story has been driven by generics and cost competitiveness. But the future of medicine is moving rapidly toward biological and personalized therapies. The launch of Nucelion represents the sector’s evolution from chemical synthesis to biological systems, from replication to creation.
It also aligns perfectly with the government’s “Make in India” and “Atmanirbhar Bharat” ambitions in healthcare—creating homegrown capacity for frontier technologies. If executed well, Nucelion could catalyze a broader shift in the Indian ecosystem—spurring investments in vector manufacturing, cold-chain logistics, and regulatory frameworks for advanced therapies.
Building the Future: Opportunities and Challenges
The opportunity is massive, but so are the challenges. Scaling cell and gene therapy requires:
• Highly skilled bioprocess talent
• A supportive regulatory environment
• Strong collaborations with global biotech innovators
• And, most critically, a path toward affordability and patient access
If Nucelion can strike the balance between global competitiveness and domestic impact, it could set the template for a new era of Indian pharma—one rooted in innovation, not imitation.
The Bigger Picture
With this launch, Bharat Biotech is sending a strong signal: India is not just a manufacturing base—it’s an innovation ecosystem in motion. From API production to complex biologics, from vaccines to cell and gene therapies, the arc of Indian pharma is bending toward higher value, deeper science, and stronger global relevance.
The next decade will likely see India’s pharma identity redefined—from “pharmacy of the world” to “biotech innovation powerhouse.”
Editor’s Take
At MedicinMan, we see this as more than a corporate announcement. It’s a marker of mindset change—from volume to value, from followership to leadership.
The rise of companies like Nucelion will not just redefine healthcare manufacturing—it will shape how India contributes to the future of human health.
Appendix: Sources
1. The Times of India – “Bharat Biotech forays into CRDMO space with new cell & gene therapies arm Nucelion Therapeutics” (Nov 2025)
https://timesofindia.indiatimes.com/business/india-business/bharat-biotech-forays-into-crdmo-space-with-new-cell-gene-therapies-arm-nucelion-therapeutics/articleshow/125061704.cms
2. Pharma ET Healthworld – “Bharat Biotech launches Nucelion, a CRDMO for advanced cell & gene therapies”
https://pharma.economictimes.indiatimes.com/news/pharma-industry/bharat-biotech-launches-nucelion-a-crdmo-for-advanced-cell-and-gene-therapies/125050244
3. Times of India (Hyderabad edition) – “Bharat Biotech to focus on cell, gene therapies”
https://timesofindia.indiatimes.com/city/hyderabad/bharat-biotech-to-focus-on-cell-gene-therapies/articleshow/125069038.cms
4. NewKerala.com – “Bharat Biotech launches Nucelion, next-gen cell & gene therapy CRDMO”
https://www.newkerala.com/news/o/bharat-biotech-launches-nucelion-next-gen-cell-gene-therapy-crdmo-529