A quiet but revolutionary milestone may be unfolding in Indian pharmaceutical history—one that could redefine the country’s global positioning not just as a manufacturer of generics but as a birthplace of cutting-edge innovation. The spark? A first-in-class antibiotic from Wockhardt, called Zaynich (WCK 5222).
What Makes Zaynich Different?
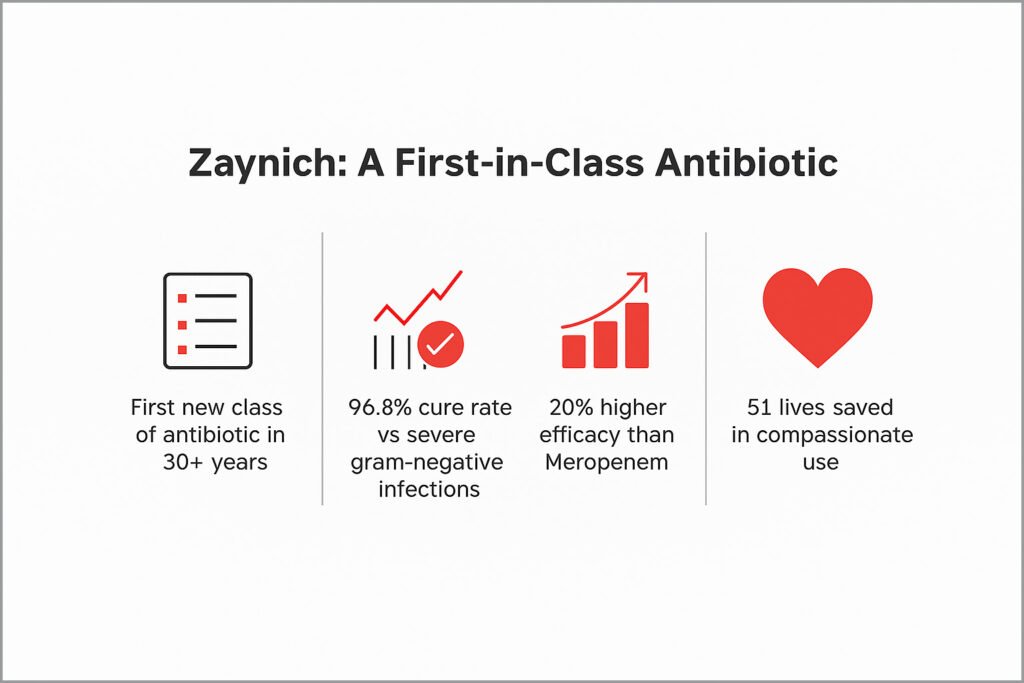
Zaynich is no incremental drug. It represents the first new class of antibiotic in over 30 years and is designed to treat life-threatening multidrug-resistant gram-negative bacterial infections, a major global health challenge.
In a large global Phase 3 clinical trial, Zaynich:
• Delivered a 96.8% clinical cure rate, with results seen within 7–10 days.
• Demonstrated 20% higher composite cure compared to the current gold standard, meropenem.
• Was effective even in compassionate use cases—saving over 51 lives, including three in the US, where all other therapies had failed.
This isn’t just another drug approval—it’s a rare clinical win in the war against antimicrobial resistance (AMR), and it’s entirely Indian in origin.
The $9 Billion Opportunity
Wockhardt estimates the global market opportunity for Zaynich to be around $9 billion.
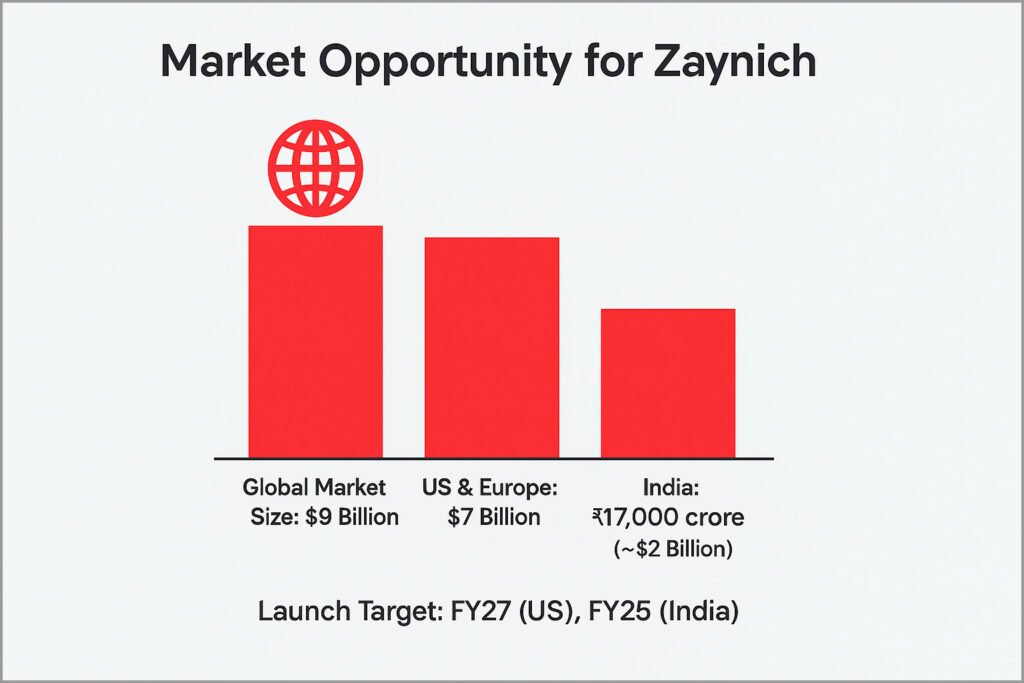
• $7 billion across the US and Europe.
• ₹17,000 crore (about $2 billion) in India alone.
With filings underway with the US FDA and India’s DCGI, the drug could launch as early as FY27 in the US and by the end of FY25 in India.
This isn’t just a blockbuster drug—it’s a statement: India can innovate at scale for global markets
Why This Is a New Dawn for Indian Pharma
1. From Generics Powerhouse to Innovation Leader
For decades, Indian pharma has been the world’s generic pharmacy—affordable, efficient, but not considered innovative.
Zaynich shifts that perception by
• Proving an Indian company can lead discovery to commercialization.
• Achieving clinical success in global trials.
• Filing with top-tier regulators like the US FDA and EMA.
2. Strategic Leadership in a Global Health Crisis
AMR is one of the greatest global health threats. Major pharma companies have stepped back from antibiotic R&D due to high costs and low commercial returns.
Wockhardt stepped in.
Zaynich’s success sends a clear message: India can lead where others retreat.
3. Trigger for Domestic Innovation Ecosystem
If Zaynich gets approved and launched successfully:
• It becomes a proof-of-concept that Indian R&D can go global.
• It will likely unlock more capital for Indian deep science and biotech startups.
• Government and the private sector may begin prioritizing antibiotic discovery and development once again.
4. Inspiration for the Next Generation
Zaynich will inspire Indian researchers, companies, and institutions to dream bigger.
To take risks.
To move beyond generics and into novel therapeutics that tackle real, global problems.
What Lies Ahead?
• The US NDA filing is expected in Q2 FY26, with a potential launch in FY27.
• India launch could happen as early as H2 FY25, pending DCGI approval.
• If successful, Zaynich could change clinical practice, patient outcomes, and India’s standing in the innovation hierarchy of global pharma.
Final Thoughts
It offers a rare combination of clinical brilliance, strategic timing, and geopolitical relevance. It shows that India is ready not just to manufacture medicine but to invent it.
And if this momentum is seized—by investors, regulators, academia, and government—Zaynich may well mark the beginning of a new era for Indian pharma.