Impact of Trump’s 100% tariff on imported patented/innovative drugs and its implications for Indian pharma

- Trump announced a 100% tariff on branded/patented finished drugs not manufactured in the U.S., with exemptions only for those building U.S. manufacturing capacity.
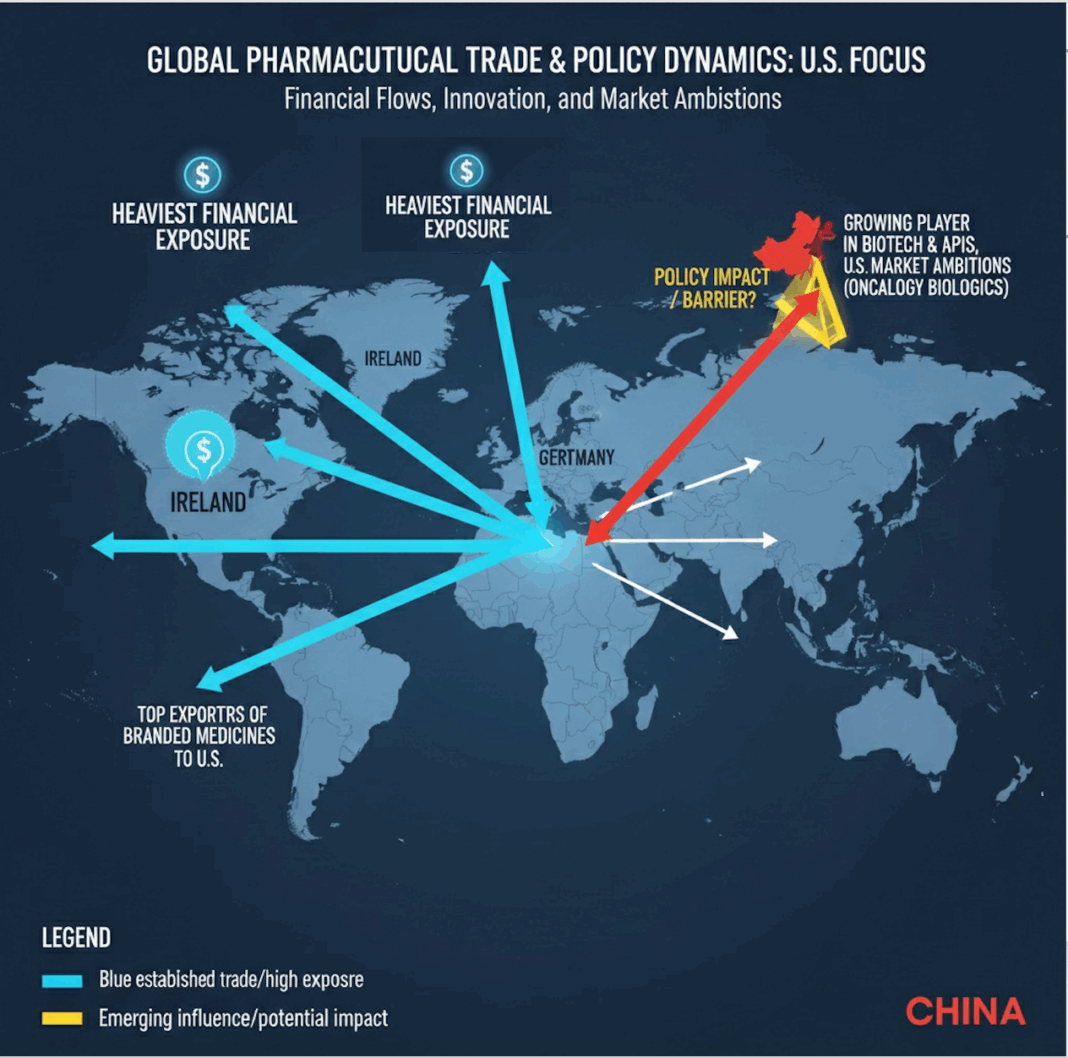
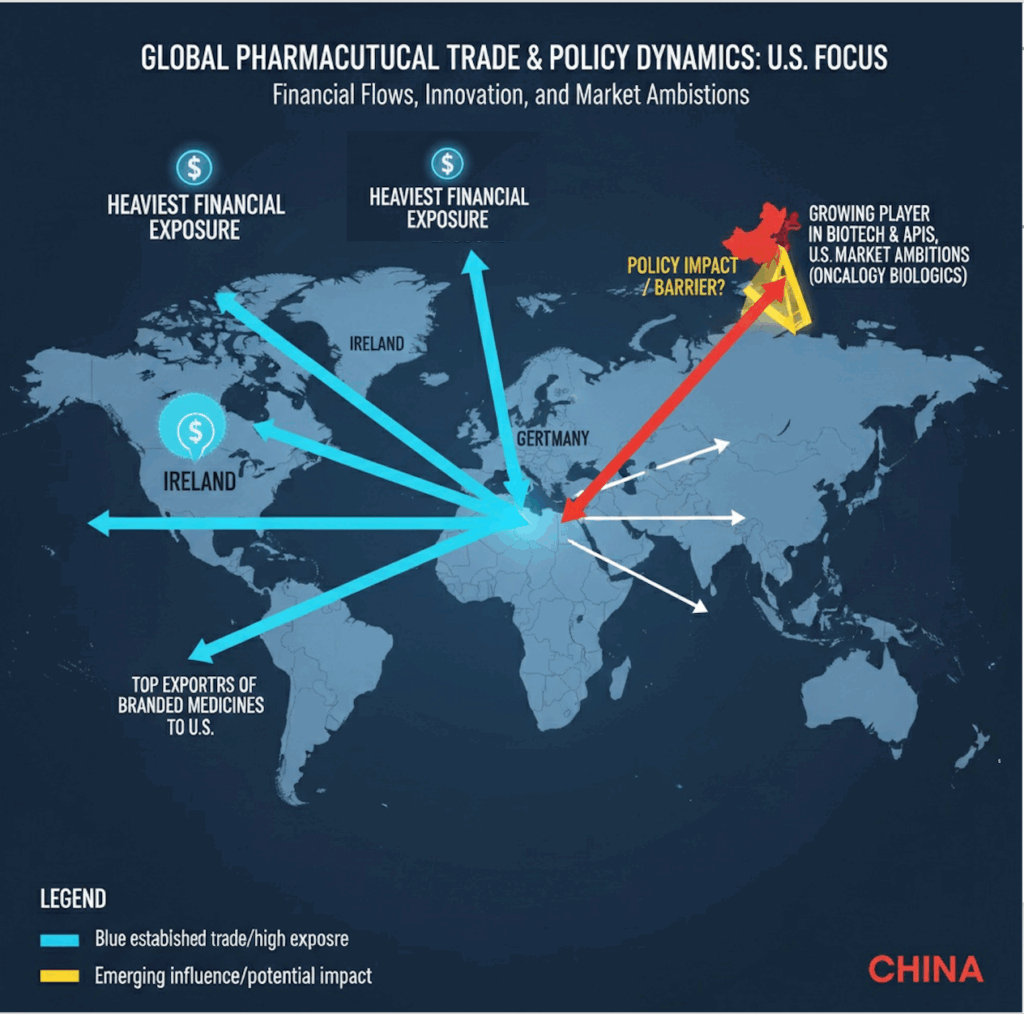
- While the U.S. remains the hub for drug approvals, innovation is increasingly global with Europe hosting major production hubs and China rising fast in biotech discovery and licensing.

- The heaviest financial exposure lies with Ireland, Switzerland, and Germany, which are top exporters of branded medicines to the U.S.
- Is the policy meant to keep China at bay? China is a growing player in biotech innovation and APIs but less dominant in exporting finished branded drugs; still, its U.S. market ambitions (especially in oncology biologics) will be hit.
- Will it impact India’s generics base? India’s main U.S. exports are generics (less affected by this tariff), but future biosimilars, specialty generics, and licensed innovative drugs from China or elsewhere could face barriers.
- This could put licensing & acquisitions at risk. Indian companies licensing Chinese or European innovations for the U.S. market will be forced to either manufacture locally in the U.S. or lose competitiveness under tariffs.

- This is policy déjà vu. Many countries (including India, China, Europe) have pushed for local pharma manufacturing over the past 15 years, but the U.S. move is disruptive because it uses tariffs as a blunt weapon.
- Net effect on India? Not a knockout blow today, but a serious challenge to Indian pharma’s aspiration to climb up the value chain into branded biologics and novel therapies in the U.S.
To read the full bog by Salil Kallianpur: https://mypharmareviews.substack.com/p/us-slaps-a-100-tariff-on-brandedpatented