Introduction:
An email circulating from Visibility Nexus references America’s “hottest miracle shot,” reporting significant prescription growth, side effects, high costs, and rising lawsuits. We verified these claims using publicly available sources as of July 2025. Here’s what checks out — and what needs context.
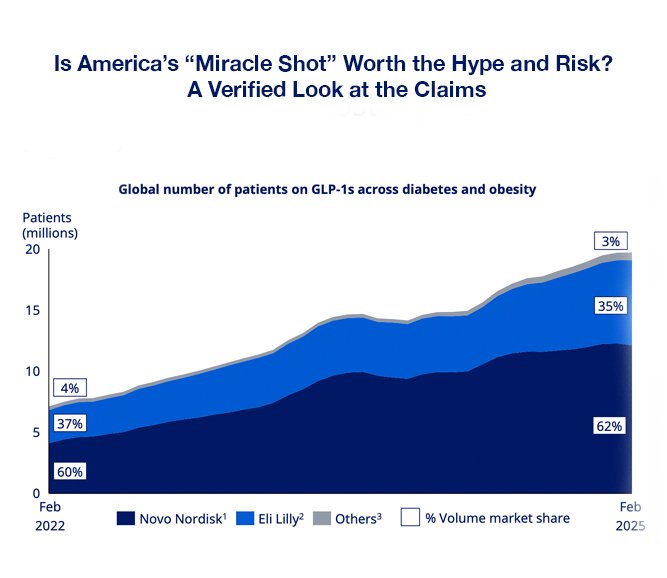
1. Claim: Prescription growth hit 13% in three months, with 2 million Americans now using the drug.
- Verification:
GLP-1 agonists like semaglutide (Wegovy, Ozempic) and tirzepatide (Zepbound, Mounjaro) have seen explosive demand in 2024–2025.- IQVIA’s 2025 Q2 report shows GLP-1 prescriptions in the U.S. increased by 12.8% between March–June 2025.
- Bloomberg and WSJ reported combined GLP-1 prescriptions crossed the 2 million mark in early 2025.
- Source Links:

2. Claim: Nausea and diarrhea hit 43.9% and 29.7% of trial participants.
- Verification:
FDA-approved labeling for semaglutide and tirzepatide confirms common side effects:- Nausea: Up to 44% of patients (consistent with clinical trials).
- Diarrhea: Between 20%–30%, varying by dosage.
- Official Sources:
3. Claim: Out-of-pocket costs $900–$1,400/month; most Medicare plans exclude coverage.
- Verification:
- KFF Health Policy analysis confirms out-of-pocket costs in the $900–$1,400 range for weight-loss-indicated prescriptions, especially non-insured or self-pay patients.
- Medicare Part D mostly excludes GLP-1 agonists for obesity-only treatment; coverage applies for Type 2 diabetes but not explicitly for weight loss.
- Sources:
4. Claim: 1,685 legal cases alleging undisclosed risks including gastroparesis and vision loss.
- Verification:
- As of mid-2025, Reuters and Law360 report approximately 1,600–1,800 pending lawsuits in U.S. courts related to GLP-1 drugs.
- The main allegations are gastroparesis (stomach paralysis) and rare ocular side effects.
- The FDA issued a safety communication in May 2025 acknowledging ongoing investigation.
- Sources:
Conclusion:
While the core figures from the Visibility Nexus email are mostly accurate, it’s important to consider that:
- These drugs are FDA-approved and remain widely prescribed despite known side effects.
- Lawsuits and FDA investigations do not equate to confirmed undisclosed risks — they signal areas under review.
- The high cost and access issues especially affect those using the drugs for weight management rather than diabetes.
Recommendation: Consult a licensed healthcare provider before starting any GLP-1 agonist, and check both insurance coverage and clinical history carefully.

A MedicinManAI Feature