Pharmaceutical companies must contend with challenges from supply chain lapses (theft, diversion, S.O.P. deviations, product recalls, reverse logistics etc.) counterfeiting and stringent regulations. These challenges get compounded when dealing across the states and country business operations, besides that not only impact tangible profits but also the intangible brand credibility. In this context, there is also a credible increase in public awareness about the genuineness of medicine (in particular medicine that require cold chain) and their predictability of clinical outcomes.
Providing visibility and full traceability becomes a paramount importance to both the Industry and the Govt. – A fool proof solution not only brings transparency in the system, but can also be a key differentiator, and undoubtedly can create immense opportunities for a competitive advantage.
Directorate General of Foreign Trade (DGFT) on 10th Jan 2011, issued a public notice announcing the implementation of a track and trace system incorporating barcode technology as per GS1 standards for all drugs and pharmaceutical products exported from India. However, the Indian domestic market for reasons unknown to us, has lagged in its implementation.
The recent notification by Govt1 dated 14th Jan 2019 has now made it mandatory for all medicines procured under Public Procurement to exhibit or display the Barcode / QR Code at primary level packaging – A right step in the right direction, which I presume has immense applicable advantages.
Track and Trace (T&T) technologies ensure continuity across drug distribution supply chains by providing visibility to all stakeholders- where the product is, and under what condition, at any given time. Such a process execution ensures a unique identity to each stock unit during its manufacturing process, and eventually through the supply chain, up to the end user. This process of checkpoints in all stages is called the “Track and Trace” system. However, Information can be accessed in the form of a unique pack coding, and through a secured database.
T&T systems rely on serialization, the assigning of unique identification numbers to products. Those products that lack identification numbers, or products with identification numbers that cannot be accounted for throughout the distribution chain, must be treated as doubtful or unreliable and removed from the market, including those coming from licensed manufacturers. The unique identifier may be stored in a barcode, electronic product code, radio frequency chip or it may be a long-digit serial number.
By identifying every product with a globally unique product number (Global Trade Item Number [GTIN]), and by capturing information on its expiry date, batch/lot number, and unique serial number (where ever applicable) allows the product’s lifecycle to be tracked from production to distribution across borders, all the way to its dispensation to patients at the drugstore or hospital.
Advantages offered by the track and trace system are:
- A reduction in medication errors
- Automated pharmacy billing
- Effective control of inventory
- Effectiveness in product recalls
- Detection of theft and product diversion
Linkage with Value added Services (VAS) – Proposed
Healthcare Distribution Management Association (HDMA) and others have been supportive of efforts to develop standard practices for serializing pharma packages with barcodes or other unique identifiers. Utilization of electronic methods allows for the reduction/elimination of human input error and thus speeds up the reconciliation and crediting process. The ability to fully automate and standardize electronic communications related to returns can lead to improvements in time efficiency, accuracy, and flexibility.
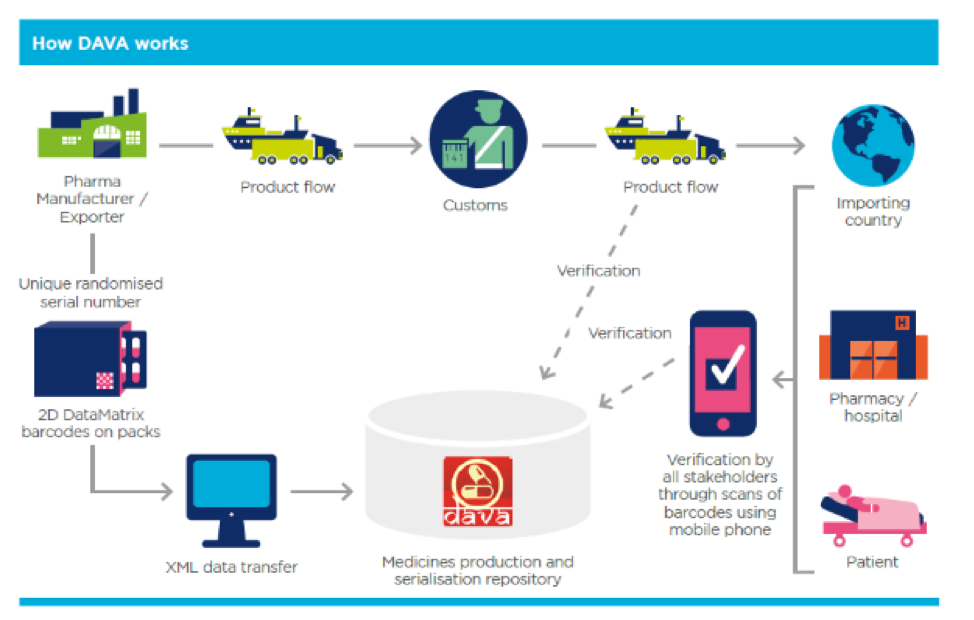
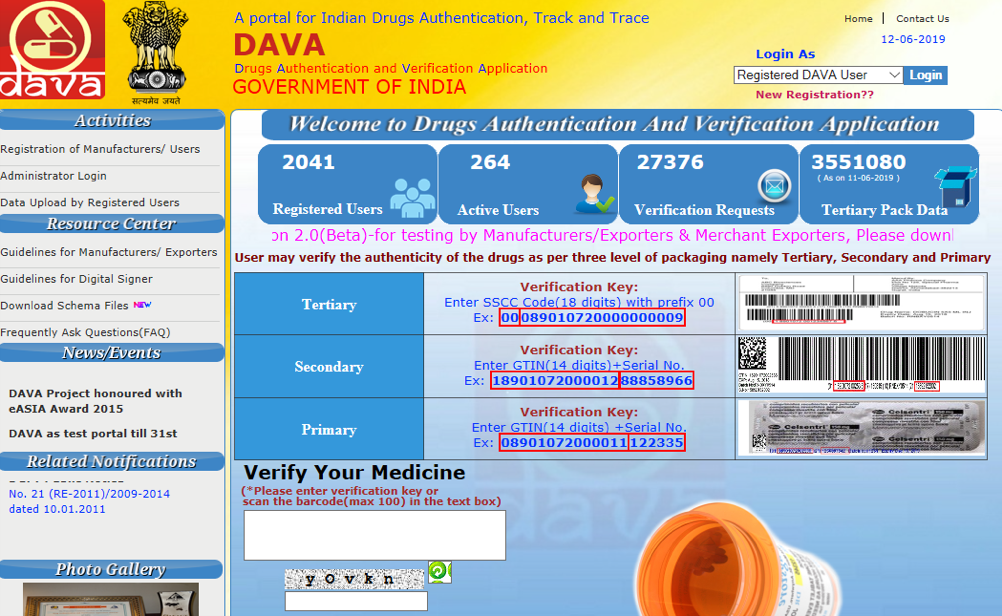
DAVA – The current traceability system available for use in India is named DAVA2, which means “medicine” in the Indian language (and is also the abbreviation for Drug Authentication and Verification Application). Designed by National Informatics Centre (NIC), DAVA is based on GS1 standards, that makes it possible to uniquely identify, capture and share important information about exported consignments with Regulators and patients across the world. This system has made it possible to gain real-time visibility to pharmaceuticals produced and exported from India. However, the same can be extended or a separate version (DAVA 2.0) can be created for extended applicability for the INDIAN domestic market.
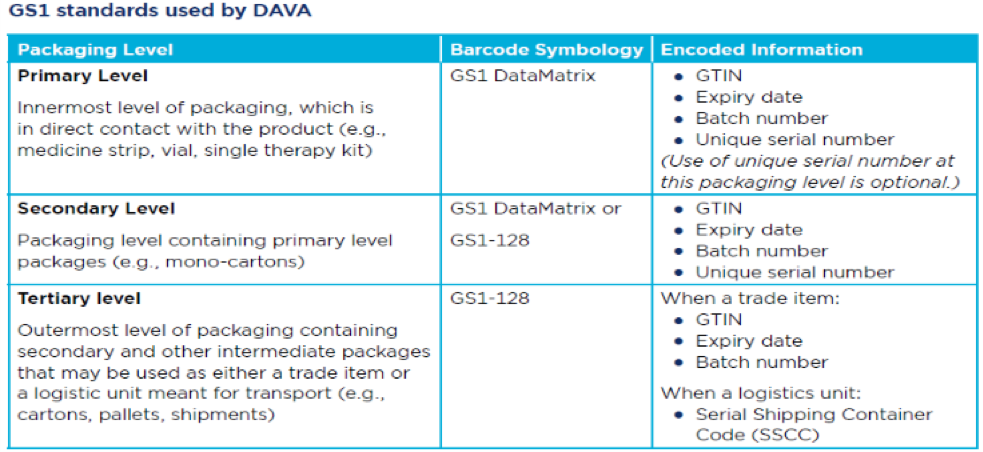
DAVA relies on the use of Global Trade Item Numbers (GTINs) plus serial numbers by manufacturers to easily identify the various packaging hierarchy levels of pharmaceuticals such as primary, secondary and tertiary (when a trade item) levels. DAVA has a mobile app version as well (1000+installs as on date) . Download the DAVA mobile app for Android.
Manufacturers maintain a parent-child relationship information (i.e. which are the products in a secondary pack and in which are the ones relatively in a tertiary pack) so that at any given point, it is possible to identify the secondary pack from that of a tertiary unit and the primary pack from the secondary unit. This information is essential to establish the pedigree of the relevant product, culminating in its validation.
Manufacturers and exporters directly upload the data to DAVA after production, prior to the consignment leaving its manufacturing facility. The accuracy, completeness and timely upload of the data is Manufacturer’s responsibility. DAVA is further integrated with a mobile application which empowers the custom officials, regulators, importers, and patients to authenticate products of products by simply scanning the barcode on any of its packaging levels. When the product barcode is scanned, eventually, all the information associated with the product is retrieved from the DAVA system. This gives the user the opportunity to authenticate or validate the product.
DAVA 2.0 for Domestic Indian Market: Proposed Applicability.
The domestic rollout of (DAVA 2.0) may have added capabilities that can have a profound impact on the Industry / Govt and End Users i.e. Patients, and through its applicability can be extended to build excellence in execution of the process. Blockchain technology can be used to airtight the system from any leakages.
“Every time the medicine changes hands, the unique number (generated at the manufacturing stage) is tracked. When the consumer gets the drug, there is a QR code or a barcode on it… you can open up an app, and you can check the entire details of where it was manufactured and also at various levels did the product exchanged hands, up to its logical destination. ” Notwithstanding the fact, once the product is sold, the code gets irrevocably audited on the blockchain that a specific product ID has been sold, and no longer exist,”
Suggested Applicability
- A) INDUSTRY: By Tracking the movement of pharmaceuticals from point of manufacture to point of sale, thus by involving all distribution points and stakeholders in the supply chain, one can monitor the availability of stocks in an area or with wholesalers or with any retailers, hospital pharmacy at any given point of time thus facilitating:
- Real-time Inventory Management
– Sales Trends and Management (Primary Vs Secondary)
– Sales Forecasting in real-time
- Differentiating billing and supply to Institutional (Public Procurement / Govt Tender Supply) from retail sale.
- Product recalls
- Execution of trade offers (Discounts and Schemes) for bar coded batch
- B) Govt: In addition to ensuring safe drugs to their country fellows the DAVA2.0 system can be integrated with E- commerce platforms like GST and E-Challan, thereby eventually supporting directly or indirectly the Govt officials for Tax validation.
- C) Patients: Many companies are finding innovative ways like patient adherence and assistance programs to create differential in the market/ fight competition or attempt to increase compliance and adherence. The T&T system provides opportunities for marketers to link bar coding / Serialization with company value assisted programs which can be accessed and monitored through the mobile app.
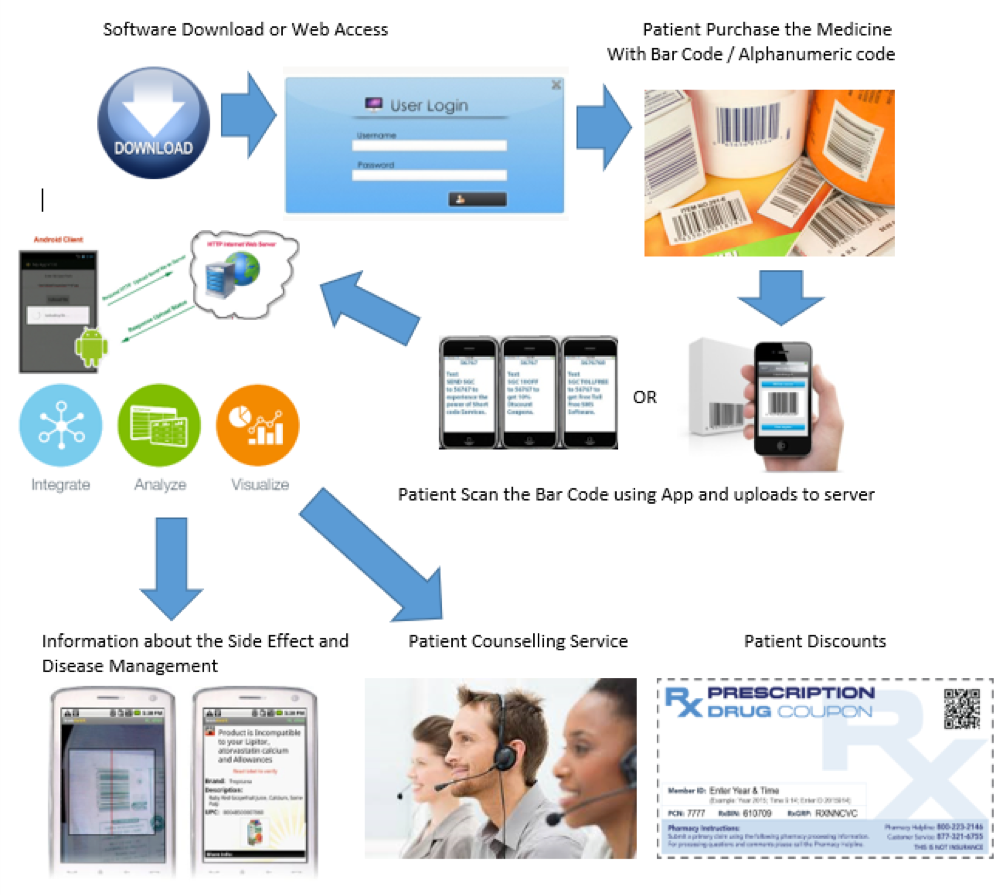
Hypothetical Workflow of Value-Added Services linked with Barcoding/Serialization
- Patients must download the company app with an in built bar code scan function on their smartphone (The access can be further linked with the social profile of the patient like logging in through Fb or google+ for authentication) or can log in to the company site and can register with his mobile phone number.
- Once the purchase is made, the patient scans the barcode on the pack with the app or can SMS the serial code to a toll-free number using an SMS code.
- Alternatively to ensure the authenticity and proof of purchase, the bar code/ serial code can be covered with a sticker or scratch guard (after purchase peel off the sticker or scratch code to unveil the barcode or alphanumeric code) so that only after the point of purchase will the patient be able to scan the item.
- Once the patient scans the barcode through his smart application, the information is captured against his account in the cloud database server or alternatively, if the patients logs on to the website or SMS’s the alphanumeric code from his phone, the same is captured against his account in the cloud base server.
- The process is repeated for all purchases made as he continues his medicine [The number of barcode scanned or no. of alphanumeric code SMS accounts is directly proportional to the number of purchases made].
The data from the cloud base server can be analyzed for purchase patterns and compliance, and if required, can be linked with patient support assistance or service coupons (patients can earn and accumulate credit points for each scan / SMS of the serial number which can then be credited against services offered by third party).
Moreover, the purchase pattern and GPS tagging can further give the geographical sales data for company sales trends.
Workflow of Value-Added Services linked with Barcoding/Serialization
Such technology can further be explored to link it with new technology formats like E-prescriptions (e-Rx): An e-prescription or e-Rx is one that is sent electronically by a doctor to a patient. It is generally considered error-free and legible, unlike the handwritten prescriptions where chances of errors or modifications are high. E-prescriptions have proved beneficial for patient safety across the world. According to the United States health department, e-prescriptions, adopted in 2003 under the Medicare Modernization Act (MMA), have reduced medication errors in the country.
In India, however, e-pharmacy apps allow users to merely upload a snapshot of a prescription as opposed to the electronic version. This practice has raised questions on the authenticity of prescriptions being sent to e-pharmacy outlets through mobile apps.
With applicability of bar coding / serialization the linkage between e-Rx and medicine dispatched through online drug portal ( via e-Pharmacy) can be further linked and validated for filing and records.
References:
1. http://pharmaceuticals.gov.in/sites/default/files/PPO%20Order%2018-01-2019.pdf
2. http://dava.gov.in/
Director of Sales and Marketing at Mitra RxDx India Pvt Ltd